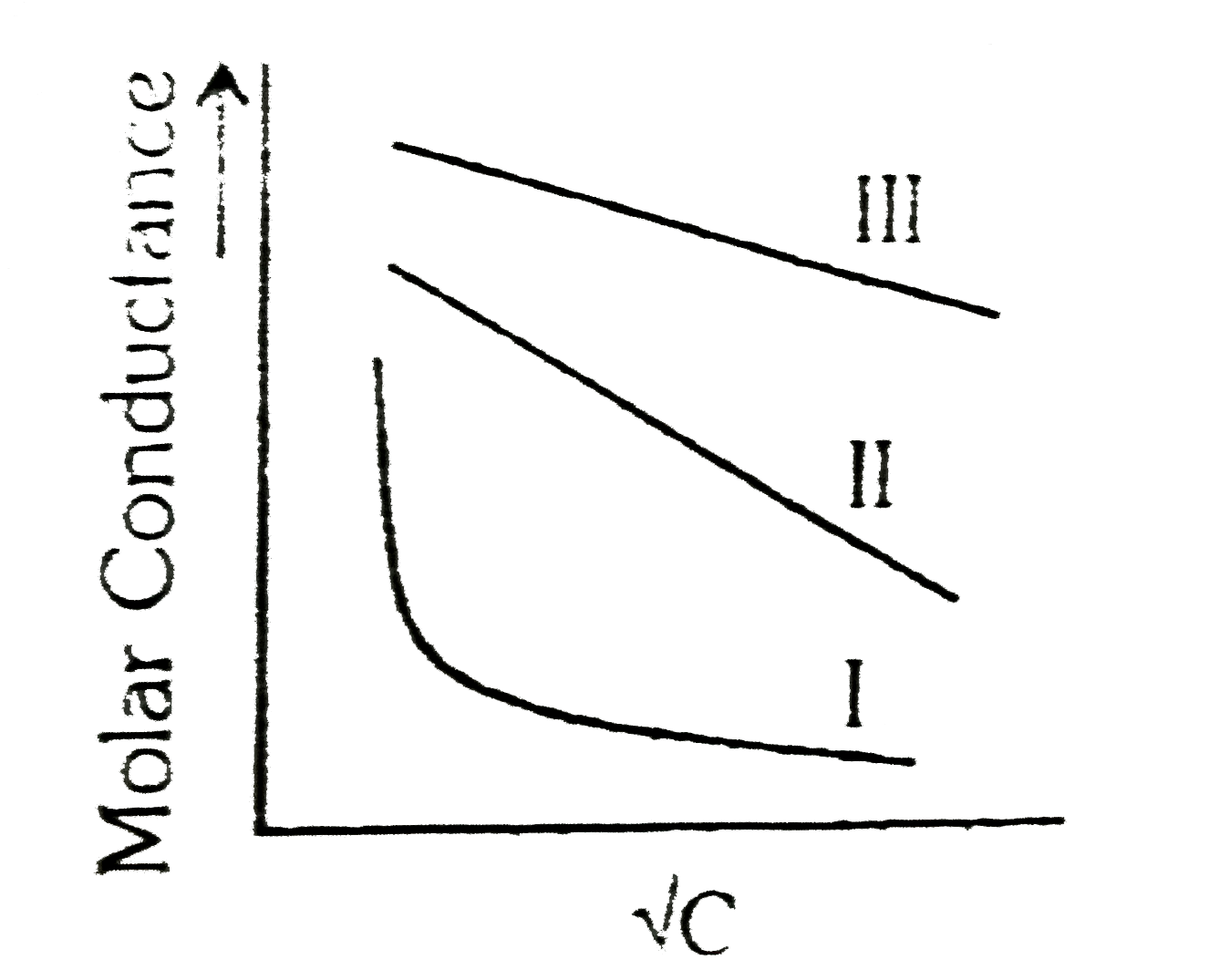
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A graph was plotted between the molar conductance of various electroly...
Text Solution
|
- Which of the following equations corrects the molar conductivity with ...
Text Solution
|
- A graph was plotted between molar conductivity of various electrolytes...
Text Solution
|
- At which of the following concentration would a solution of an electro...
Text Solution
|
- In which of the following concentrations of a particular electrolyte m...
Text Solution
|
- दुर्बल विद्युत्-अपघट्य के लिए मोलर चालकता एवं सीमान्त मोलर चालकता में ...
Text Solution
|
- Above plot represents the variation of molar conductance against sqrtC...
Text Solution
|
- The empirical relationship between molar conductance and concentration...
Text Solution
|
- Give the empirical relationship between molar conductance and concentr...
Text Solution
|