A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
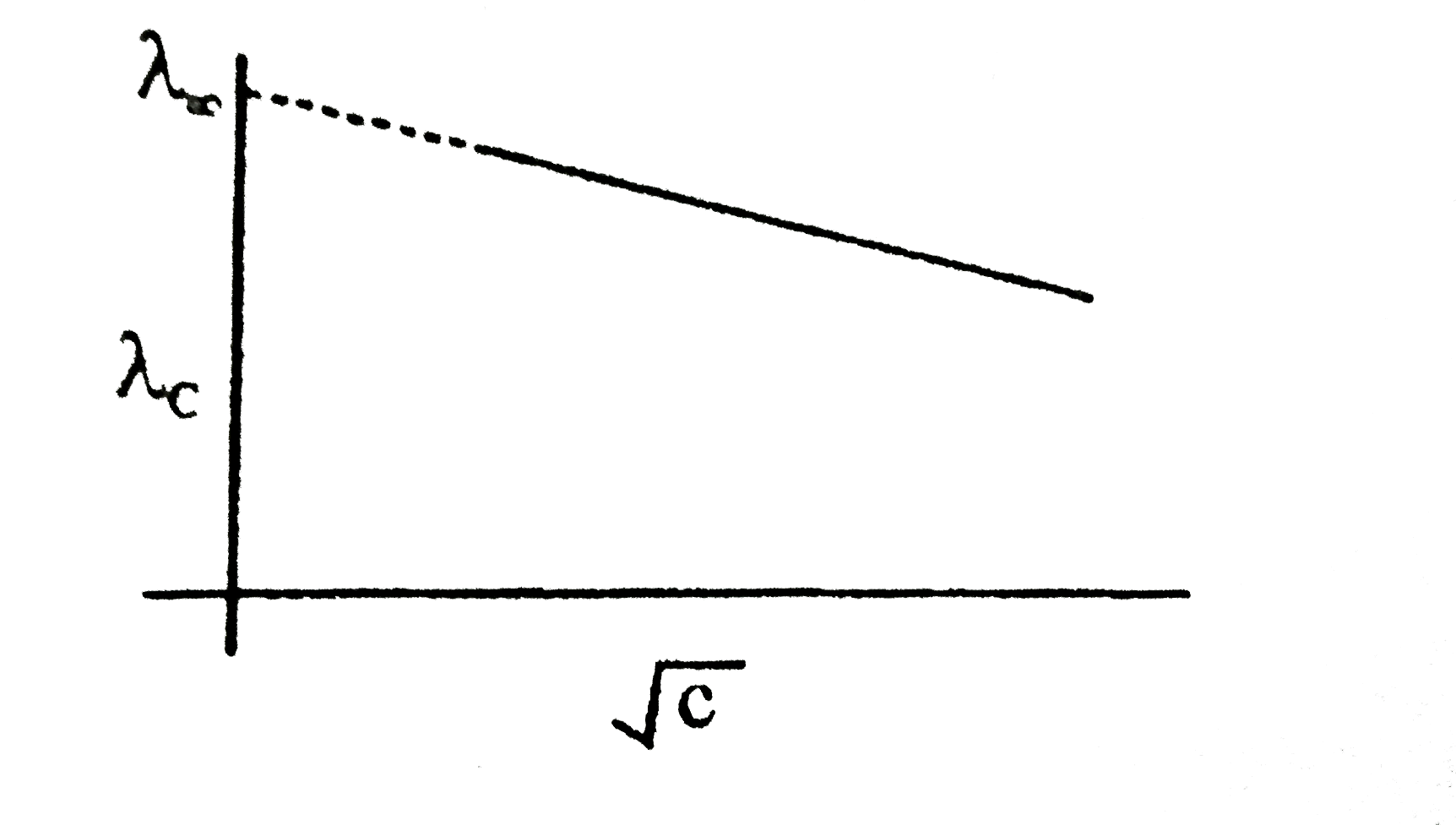
- The equivalent conductance of NaCl at concentration C and at infinite ...
Text Solution
|
- The equivalent conductance of NaCl at concentration of C and at infini...
Text Solution
|
- The equivalent conductance of NaCl at concentration C and at infinite ...
Text Solution
|
- The molar conductance of NaCl varies with the concentration as shown i...
Text Solution
|
- The molar conductance of NaCl varies with the concentration as shown i...
Text Solution
|
- At infinite dilution, the equivalent conductivity of the electrolyte i...
Text Solution
|
- The equivalent conductances of NACl at concentration c and at infinite...
Text Solution
|
- The equivalent conductance of NaCl at concentration C and at infinite ...
Text Solution
|
- The equivalent conductance of naCl at concentration C and at infinite ...
Text Solution
|