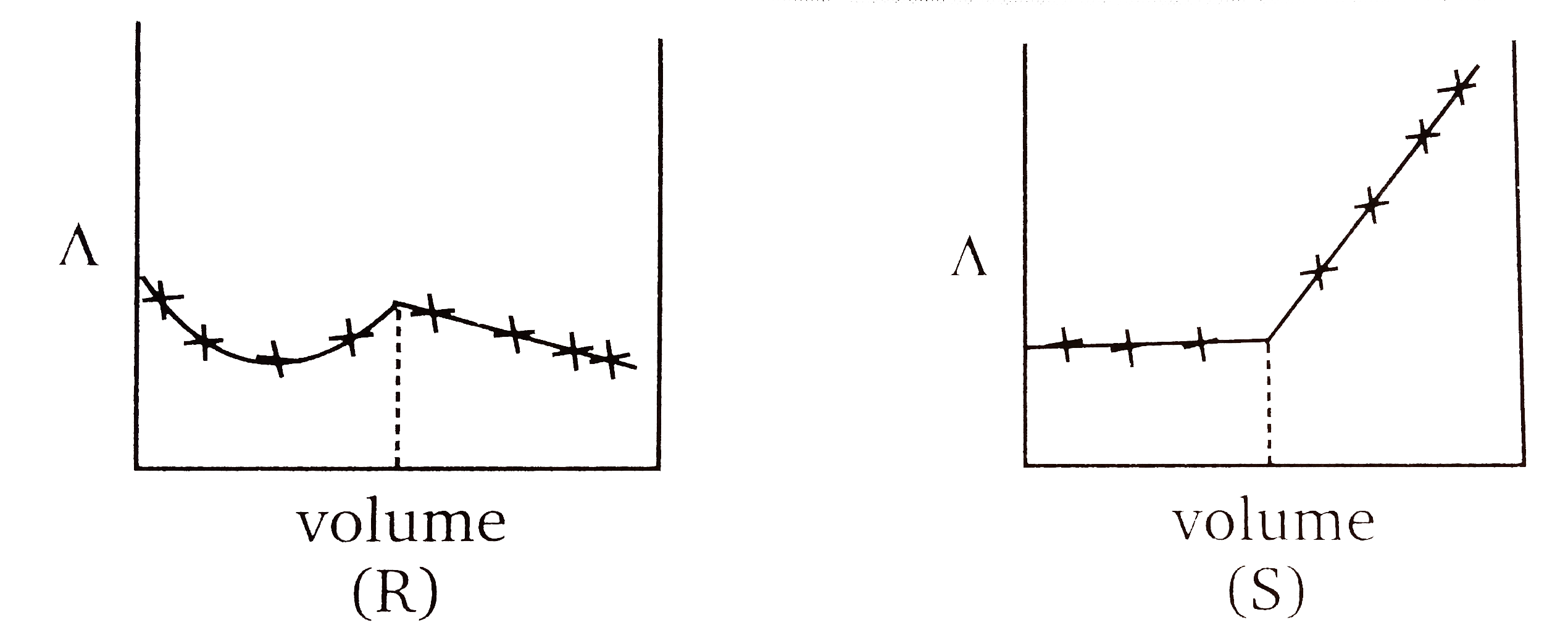
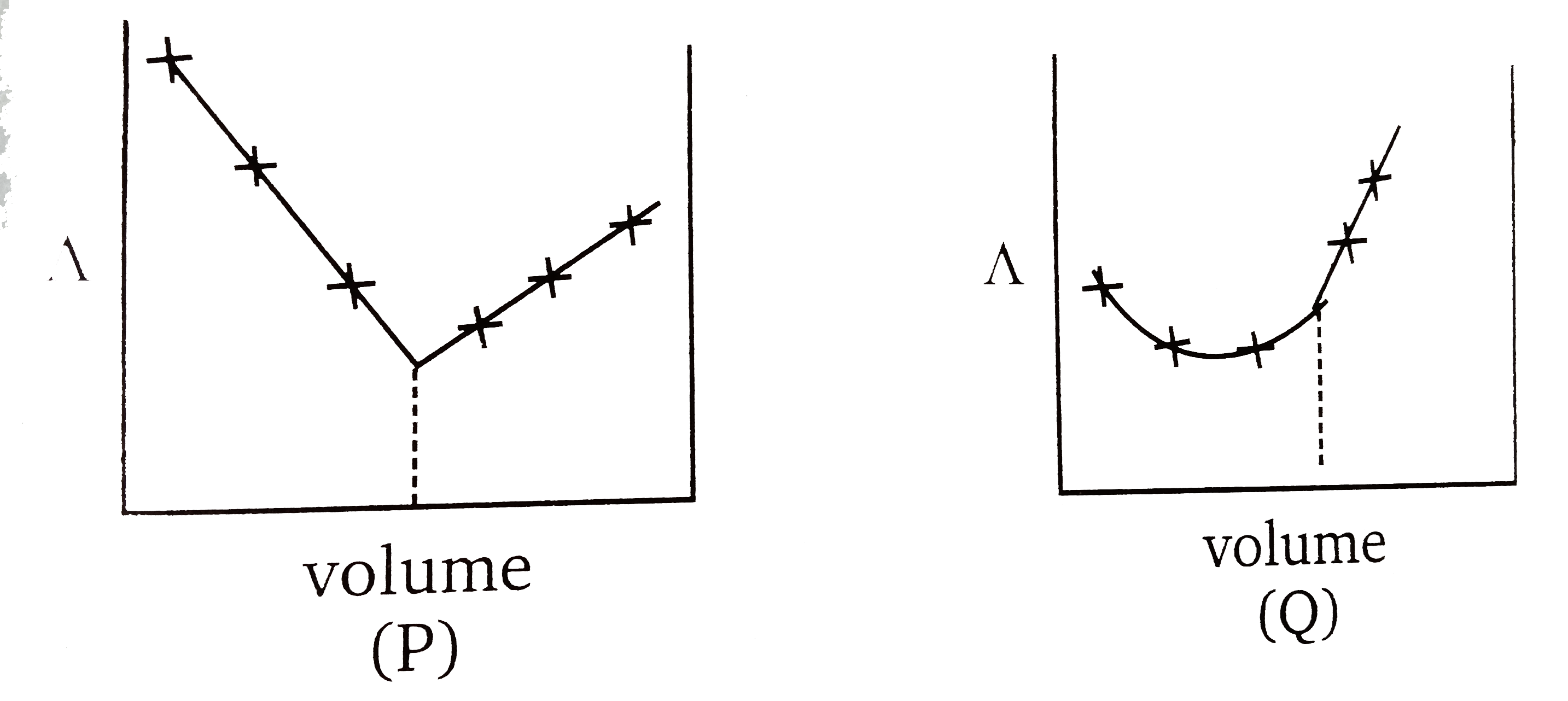A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- AgNO(3)(aq.) was added to an aqeous KCl solution gradually and the con...
Text Solution
|
- AgNO(2)(aq) was added to an aqueous KCl solution gradually and the con...
Text Solution
|
- AgNO(3)(aq.) was added to an aqeous KCl solution gradually and the con...
Text Solution
|
- To prepare a solution of concentrated of 0.03g//mlof AgNO(3),what amou...
Text Solution
|
- AgNO(3) (aq) was added to an aqueous KCl solution gradually and the co...
Text Solution
|
- AgNO(3)(aq) was added to an aqueous KCl soltuion gradually and conduct...
Text Solution
|
- AgNO(3(aq) was added to an aqueous KCl solution gradully and the condu...
Text Solution
|
- Type of collcidal solutions result when AgNO(3) Solution is added to...
Text Solution
|
- The molar conductivity of a 0.5 mol//dm^(3) solution of AgNO(3) with e...
Text Solution
|