A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
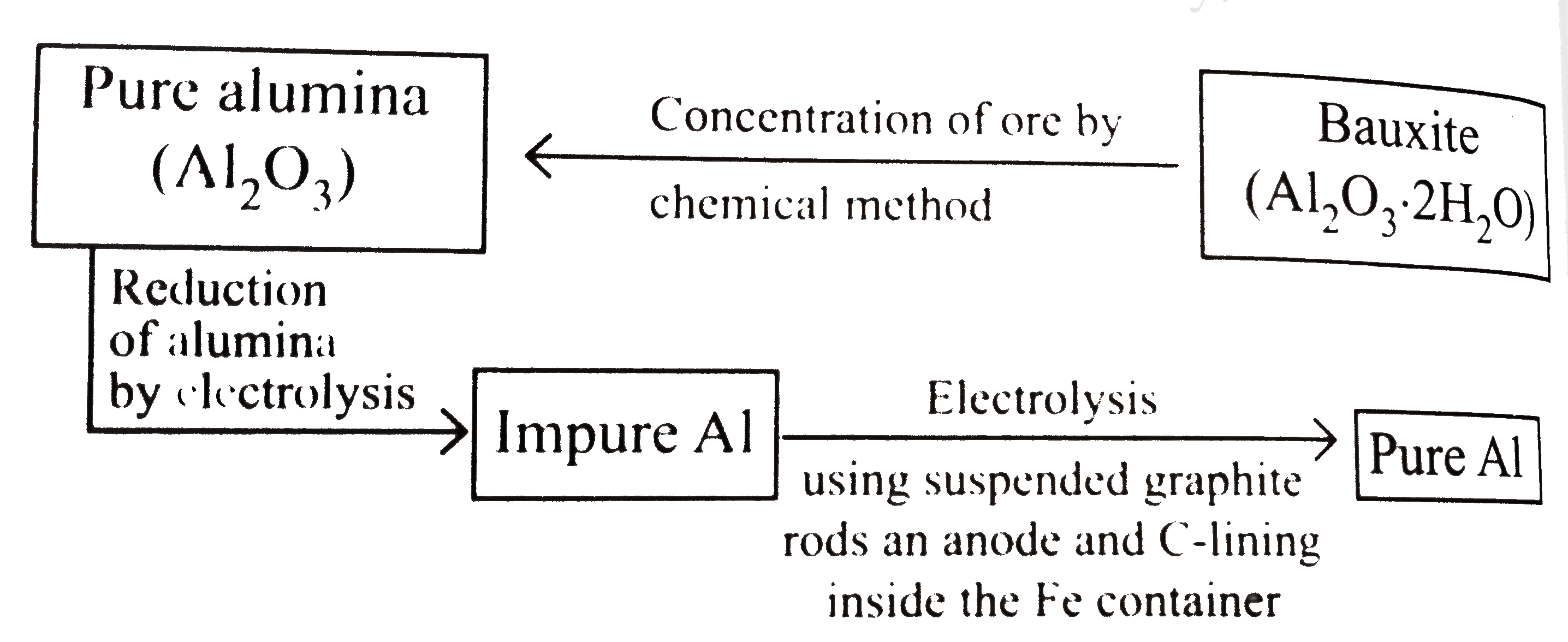
- Extraction of aluminium can be understood by : Electrolytric redu...
Text Solution
|
- Extraction of aluminium can be understood by : Electrolytric redu...
Text Solution
|
- Extraction of aluminium can be understood by : Electrolytric redu...
Text Solution
|
- Extraction of aluminium can be understood by : Electrolytric reduction...
Text Solution
|
- Extraction of aluminium can be understood by : Electrolytric reduction...
Text Solution
|
- Electrolytic reducation of Al(2)O(3) Electrolyte (Al(2)O(3)+cryolite) ...
Text Solution
|
- Extraction of aluminium can be understood by : Electrolytric redu...
Text Solution
|
- Extraction of aluminium can be understood by : Electrolytric redu...
Text Solution
|
- Extraction of aluminium can be understood by : Electrolytric redu...
Text Solution
|