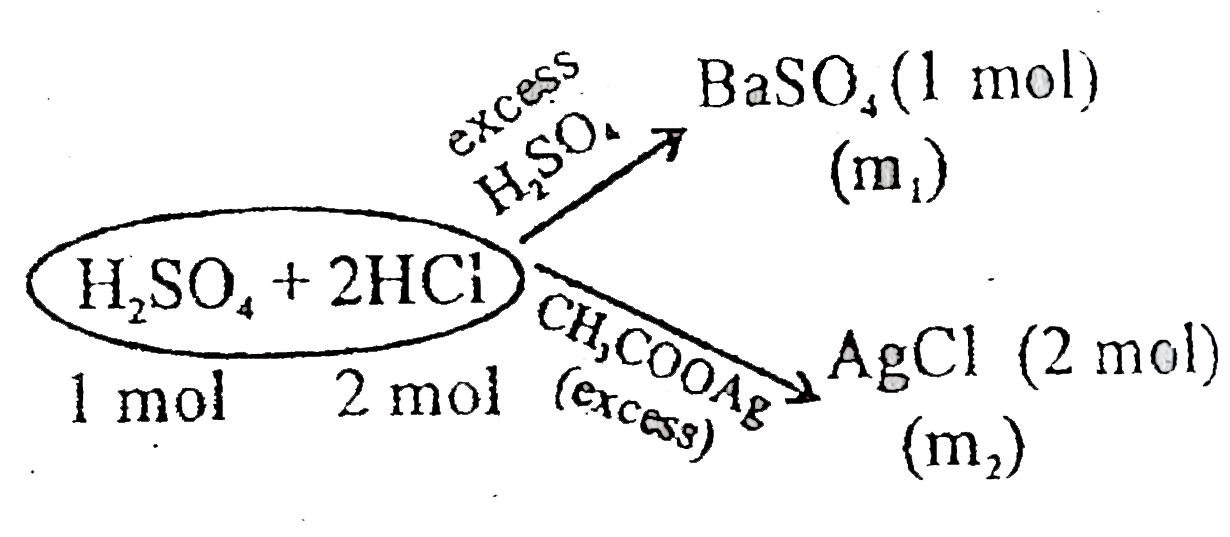A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- SO(2)Cl(2) is dissolved in water to obtain H(2)SO(4) and HCL. In two ...
Text Solution
|
- Sulphuryl chloride SO(2)Cl(2) reacts with water to give a mixture of H...
Text Solution
|
- The integral enthalpy of solution in kJ of one mole of H(2)SO(4) disso...
Text Solution
|
- SO(2)Cl(2) is dissolved in water to obtain H(2)SO(4) and HCL. In two ...
Text Solution
|
- 50 mL solution of BaCl(2) (20.8% w//v) and 100 mL solution of H(2)SO(4...
Text Solution
|
- SO(2)Cl(2), (sulphuryl chloride) reacts with water to give a mixture o...
Text Solution
|
- When a equimolar mixture of Cu(2) S and CuS is tirated with Ba(MnO(4))...
Text Solution
|
- One commercial system removes SO(2) emission from smoke at 95(@)C by t...
Text Solution
|
- Sulphuryl chloride, SO(2)Cl(2) , reacts with H(2)O to give mixture of ...
Text Solution
|