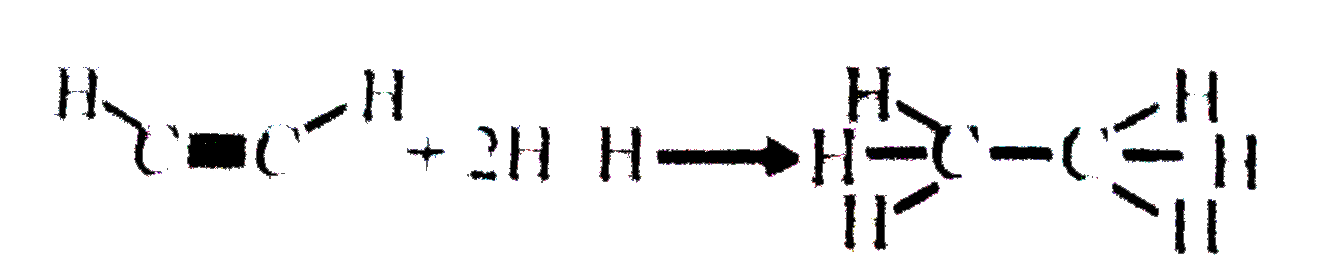A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Recommended Questions
- From the following bond energies :- H-H "bond energy" = 420 KJmol^(-...
Text Solution
|
- From the following bond energies: H--H bond energy: 431.37KJmol^(-1) C...
Text Solution
|
- From the following bond energies :- H-H "bond energy" = 420 KJmol^(-1)...
Text Solution
|
- From the following bond energies H-H bond energy 431.37 kJ mol^(-1) ...
Text Solution
|
- From the following bond energies H-H bond energy 431.37 kJ mol^(-1) ...
Text Solution
|
- Using the data provided, calculate the multiple bond energy (kJ mol^(-...
Text Solution
|
- Calculate Delta H ^(@) for the reaction CH(2)= CH(2) + 3O(2) rarr 2CO...
Text Solution
|
- निम्न अभिक्रिया की एन्थैल्पी की गणना कीजिये । CH(2) = CH(2)(g) + H(2...
Text Solution
|
- From the following bond energies: H-H bond enerwgy: 431.37 kJ mol^(-1)...
Text Solution
|