Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN -GEOMETRICAL OPTICS-EXERCISE - 04 (A)
- A closed container of volume 0.02m^3contains a mixture of neon and arg...
Text Solution
|
- An oxygen cylinder of volume 30 litres has an initial gauge pressure o...
Text Solution
|
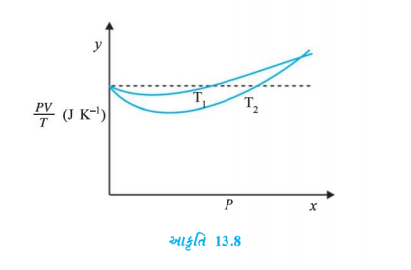
- Figure Show plot of PV//T versus P for 1.00xx10^(-3) kg of oxygen gas ...
Text Solution
|
- For a gas (R)/(C(P)) = 0.4. For this gas calculate the following (i) A...
Text Solution
|
- One gram mole of oxygen at 27^@ and one atmospheric pressure is enclos...
Text Solution
|
- An ideal gas in enclosed in a tube and is held in the vertical positio...
Text Solution
|
- Two moles of helium gas undergo a cyclic process as shown in Fig. Assu...
Text Solution
|
- Examine the following plots and predict whether in (i) P(1) lt P(2) "a...
Text Solution
|
- A sample of 2 kg monoatomic helium (assumed ideal ) is taken from A to...
Text Solution
|
- In the given figure an ideal gas changes its state from A to state C b...
Text Solution
|
- The pressure in monoatomic gas increases linearly from =4xx10^(5) Nm^...
Text Solution
|
- On mole of a monoatomic ideal gas is taken through the cycle shown in ...
Text Solution
|
- At 27^@C two moles of an ideal monoatomic gas occupy a volume V. The g...
Text Solution
|
- Three moles of an ideal gas (Cp=7/2R) at pressure, PA and temperature ...
Text Solution
|
- An ideal gas having initial pressure p, volume V and temperature T is ...
Text Solution
|
- Two moles of helium gas (gamma = 5 / 3) are initially at 27^@C and occ...
Text Solution
|
- An ideal gas has a specific heat at constant pressure CP=(5R)/2. The g...
Text Solution
|
- Calculate the work done when one mole of a perfect gas is compressed a...
Text Solution
|
- A gaseous mixture enclosed in a vessel of volume V consists of one gra...
Text Solution
|
- An ideal gas is taken through a cyclic thermodynamic process through f...
Text Solution
|