A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN -GEOMETRICAL OPTICS-EXERCISE - 05 (A)
- A liquid in a beaker has temperature theta(t) at time t and theta0 is ...
Text Solution
|
- If a piece of metal is heated to temperature theta and the allowed to ...
Text Solution
|
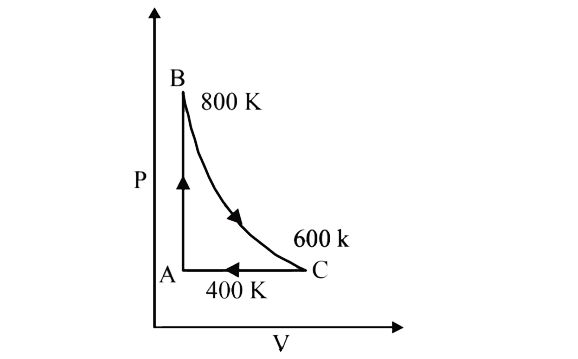
- One mole of a diatomic ideal gas undergoes a cyclic process ABC as sho...
Text Solution
|
- Three rods of Copper, Brass and Steel are welded together to from a Y ...
Text Solution
|
- Cooking gas container are kept in a lorry moving with uniform speed. T...
Text Solution
|
- At what temperature is the rms velocity of a hydrogen molecule equal t...
Text Solution
|
- 1 mole of a gas with gamma=7//5 is mixed with 1 mole of a gas with gam...
Text Solution
|
- During an adiabatic process, the pressure of gas is found to be propor...
Text Solution
|
- One mole of ideal monoatomic gas (gamma=5//3) is mixed with one mole o...
Text Solution
|
- A gaseous mixture consists of 16g of helium and 16 g of oxygen. The ra...
Text Solution
|
- If C(p) and C(v) denoted the specific heats of unit mass of nitrogen a...
Text Solution
|
- An insulated container of gas has two chambers separated by an insulat...
Text Solution
|
- Velocity of sound in O(2) gas is 460 ms^(-1) at certain temperature. ...
Text Solution
|
- One kg of a diatomic gas is at pressure of 8xx10^4N//m^2. The density ...
Text Solution
|
- 100g of water is heated from 30^@C to 50^@C. Ignoring the slight expan...
Text Solution
|
- Three perfect gases at absolute temperature T(1), T(2) and T(3) are mi...
Text Solution
|
- The specific heat capacity of a metal at low temperature (T) is given ...
Text Solution
|
- A container with insulating walls is divided into two equal parts by a...
Text Solution
|
- Which statements is incorrect?
Text Solution
|
- If mass-energy equivalence is taken into account , when water is coole...
Text Solution
|