The figure below shows the variation of specific heat capacity (C) of a solid as a function of temperature (T). The temperature is increased continuously form 0 to 500K at a constant rate. Ignoring any volume change, the following statement (s) is (are) correct to a reasonable approximation.
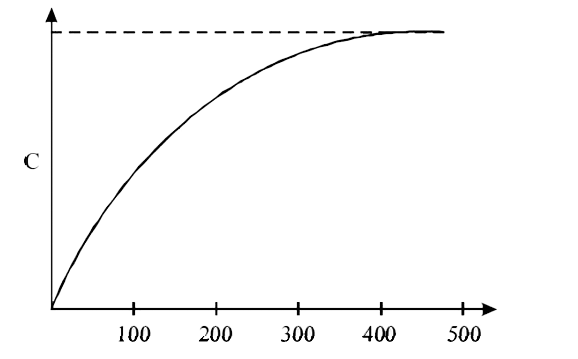
The figure below shows the variation of specific heat capacity (C) of a solid as a function of temperature (T). The temperature is increased continuously form 0 to 500K at a constant rate. Ignoring any volume change, the following statement (s) is (are) correct to a reasonable approximation.


A
The rate at which heat is absorbed in the range `0-100K` varies linearly with temperature `T`.
B
Heat absorbed in increasing the temperature from `0-100K` is less than the heat required for increasing the temperature from `400-500K`.
C
There is no change in the rate of heat absorption in the range `400-500K`
D
The rate of heat absorption increases in the range `200-300K`
Text Solution
Verified by Experts
The correct Answer is:
A, B, C, D
(A) From `0"to"100`k the major part of graph lies in linear region and very small part in non-linear region, therefore to a reasonable approximation between `0 K - 100K`, graph of `C` vs `T`is linear.
_E01_422_S01.png)
(B) by comparing area under curve
_E01_422_S02.png)
(C) from `400K "to" 500K` , Graph of `C` vs `T` become asymptotic hence rate of heat absorption become constant
(D) The rate of heat absorption increases as `C` is increasing.
_E01_422_S01.png)
(B) by comparing area under curve
_E01_422_S02.png)
(C) from `400K "to" 500K` , Graph of `C` vs `T` become asymptotic hence rate of heat absorption become constant
(D) The rate of heat absorption increases as `C` is increasing.
Topper's Solved these Questions
GEOMETRICAL OPTICS
ALLEN |Exercise EXERCISE - 05 (B) (MATCH THE COLUMN)|3 VideosGEOMETRICAL OPTICS
ALLEN |Exercise EXERCISE - 05 (B) (ASSERTION & REASON )|9 VideosGEOMETRICAL OPTICS
ALLEN |Exercise EXERCISE - 05 (B)|58 VideosCURRENT ELECTRICITY
ALLEN |Exercise EX.II|66 VideosGRAVITATION
ALLEN |Exercise EXERCISE 4|9 Videos
Similar Questions
Explore conceptually related problems
A well insulated substance in solid state is heated at a constant rate until it vaporizes completely. The temperature-time graph of the substance is shown below . Which of the following statements is//are true?
The straight lines in the figure depict the variations in temperature DeltaT as a function of the amount of heat supplied Q is different process involving the change of state of a monoatomic and a diatomic ideal gas . The initial states, ( P,V,T ) of the two gases are the same . Match the processes as described, with the straight lines in the graph as numbered. {:(,"Column-I",,"Column-II"),((A),"Isobaric process of monoatomic gas." ,(p),"1" ),((B),"Isobaric process of diatomic gas" ,(q),"2" ),((C),"Isochoric process of monoatomic gas" ,(r),"Minimum at section B"), ((D),"Isochoric process of diatomic gas" ,(s),"x-axis (i.e.'Q' axis)"):}
Scientists are working hard to develop nuclear fusion reactor Nuclei of heavy hydrogen, _(1)^(2)H , known as deuteron and denoted by D , can be thought of as a candidate for fusion rector . The D-D reaction is _(1)^(2) H + _(1)^(2) H rarr _(2)^(1) He + n+ energy. In the core of fusion reactor, a gas of heavy hydrogen of _(1)^(2) H is fully ionized into deuteron nuclei and electrons. This collection of _1^2H nuclei and electrons is known as plasma . The nuclei move randomly in the reactor core and occasionally come close enough for nuclear fusion to take place. Usually , the temperature in the reactor core are too high and no material will can be used to confine the to plasma for a time t_(0) before the particles fly away from the core. If n is the density (number volume ) of deuterons , the product nt_(0) is called Lawson number. In one of the criteria , a reactor is termed successful if Lawson number is greater then 5 xx 10^(14) s//cm^(2) it may be helpfull to use the following boltzmann constant lambda = 8.6 xx 10^(-5)eV//k, (e^(2))/(4 pi s_(0)) = 1.44 xx 10^(-9) eVm Assume that two deuteron nuclei in the core of fusion reactor at temperature energy T are moving toward each other, each with kinectic energy 1.5 kT , when the seperation between them is large enough to neglect coulomb potential energy . Also neglate any interaction from other particle in the core . The minimum temperature T required for them to reach a separation of 4 xx 10^(-15) m is in the range
Scientists are working hard to develop nuclear fusion reactor Nuclei of heavy hydrogen, _(1)^(2)H , known as deuteron and denoted by D , can be thought of as a candidate for fusion rector . The D-D reaction is _(1)^(2) H + _(1)^(2) H rarr _(2)^(1) He + n+ energy. In the core of fusion reactor, a gas of heavy hydrogen of _(1)^(2) H is fully ionized into deuteron nuclei and electrons. This collection of _1^2H nuclei and electrons is known as plasma . The nuclei move randomly in the reactor core and occasionally come close enough for nuclear fusion to take place. Usually , the temperature in the reactor core are too high and no material will can be used to confine the to plasma for a time t_(0) before the particles fly away from the core. If n is the density (number volume ) of deuterons , the product nt_(0) is called Lawson number. In one of the criteria , a reactor is termed successful if Lawson number is greater then 5 xx 10^(14) s//cm^(2) it may be helpfull to use the following boltzmann constant lambda = 8.6 xx 10^(-5)eV//k, (e^(2))/(4 pi s_(0)) = 1.44 xx 10^(-9) eVm Assume that two deuteron nuclei in the core of fusion reactor at temperature energy T are moving toward each other, each with kinectic energy 1.5 kT , when the seperation between them is large enough to neglect coulomb potential energy . Also neglate any interaction from other particle in the core . The minimum temperature T required for them to reach a separation of 4 xx 10^(-15) m is in the range
Scientists are working hard to develop nuclear fusion reactor Nuclei of heavy hydrogen, _(1)^(2)H , known as deuteron and denoted by D , can be thought of as a candidate for fusion rector . The D-D reaction is _(1)^(2) H + _(1)^(2) H rarr _(2)^(1) He + n+ energy. In the core of fusion reactor, a gas of heavy hydrogen of _(1)^(2) H is fully ionized into deuteron nuclei and electrons. This collection of _1^2H nuclei and electrons is known as plasma . The nuclei move randomly in the reactor core and occasionally come close enough for nuclear fusion to take place. Usually , the temperature in the reactor core are too high and no material will can be used to confine the to plasma for a time t_(0) before the particles fly away from the core. If n is the density (number volume ) of deuterons , the product nt_(0) is called Lawson number. In one of the criteria , a reactor is termed successful if Lawson number is greater then 5 xx 10^(14) s//cm^(2) it may be helpfull to use the following boltzmann constant lambda = 8.6 xx 10^(-5)eV//k, (e^(2))/(4 pi s_(0)) = 1.44 xx 10^(-9) eVm In the core of nucleus fusion reactor , the gas become plasma because of
A quantity of 2 mole of helium gas undergoes a thermodynamic process, in which molar specific heat capacity of the gas depends on absolute temperature T , according to relation: C=(3RT)/(4T_(0) where T_(0) is initial temperature of gas. It is observed that when temperature is increased. volume of gas first decrease then increase. The total work done on the gas until it reaches minimum volume is :-
Each phase of a material can exits only in certain regions of pressure and temperature . P-T phase diagrams, in which pressure is plotted versus temperature, show the regions corresponding to various phases and phase transformations . P-V diagrams, on the other hand , can be used to study pressure volume relationship at a constant temperature. If the liquid and gaseous phases of a pure substances are heated together in a closed container, both the temperature and the vapor pressure will increase until a point is reached at which the two phases can no longer be distinguished from one another. The temperature and pressure at which this occurs are called the critical temperature and pressure. Exceeding either of these parameters, by itself ,will cause the "gas"//"liguid" phase transition to disappear. if the other variable is then changed as well, while the first variable is maintained above its critical point , a gradual transition will occur between the gaseous and liquid phases, with no clear boundary.(The liquid and solid phases, on the other hand , maintain a distinct boundary at all pressure above the triple point). Shown in figure is a combined P-T phase diagram for material A and B . If heat is added to solids A and B , each in a container that is open to the atmosphere :-
Each phase of a material can exits only in certain regions of pressure and temperature . P-T phase diagrams, in which pressure is plotted versus temperature, show the regions corresponding to various phases and phase transformations . P-V diagrams, on the other hand , can be used to study pressure volume relationship at a constant temperature. If the liquid and gaseous phases of a pure substances are heated together in a closed container, both the temperature and the vapor pressure will increase until a point is reached at which the two phases can no longer be distinguished from one another. The temperature and pressure at which this occurs are called the critical temperature and pressure. Exceeding either of these parameters, by itself ,will cause the "gas"//"liguid" phase transition to disappear. if the other variable is then changed as well, while the first variable is maintained above its critical point , a gradual transition will occur between the gaseous and liquid phases, with no clear boundary.(The liquid and solid phases, on the other hand , maintain a distinct boundary at all pressure above the triple point). Shown in figure is a combined P-T phase diagram for material A and B . Which is true about the substance in figure?
A circus wishes to develop a new clown act. Fig. (1) shows a diagram of the proposed setup. A clown will be shot out of a cannot with velocity v_(0) at a trajectory that makes an angle theta=45^(@) with the ground. At this angile, the clown will travell a maximum horizontal distance. The cannot will accelerate the clown by applying a constant force of 10, 000N over a very short time of 0.24s . The height above the ground at which the clown begins his trajectory is 10m . A large hoop is to be suspended from the celling by a massless cable at just the right place so that the clown will be able to dive through it when he reaches a maximum height above the ground. After passing through the hoop he will then continue on his trajectory until arriving at the safety net. Fig (2) shows a graph of the vertical component of the clown's velocity as a function of time between the cannon and the hoop. Since the velocity depends on the mass of the particular clown performing the act, the graph shows data for serveral different masses. From figure 2, approximately how much time will it take for clown with a mass of 60 kg to reach the safety net located 10 m below the height of the cannot?
A solid body X of heat capacity C is kept in an atmosphere whose temperature is T_A=300K . At time t=0 the temperature of X is T_0=400K . It cools according to Newton's law of cooling. At time t_1 , its temperature is found to be 350K. At this time (t_1) , the body X is connected to a large box Y at atmospheric temperature is T_4 , through a conducting rod of length L, cross-sectional area A and thermal conductivity K. The heat capacity Y is so large that any variation in its temperature may be neglected. The cross-sectional area A of hte connecting rod is small compared to the surface area of X. Find the temperature of X at time t=3t_1.
ALLEN -GEOMETRICAL OPTICS-EXERCISE - 05 (B) (MCQ)
- Let overlinev , v("rms") and v("p") respectively denote the mean speed...
Text Solution
|
- During the melting of a slab of ice at 273K at atmospheric pressure :-
Text Solution
|
- A bimetallic strip is formed out of two identical strips one of copper...
Text Solution
|
- C(p) and C(v) denote the molar specific heat capacities of a gas at co...
Text Solution
|
- The figure shows the P-V plot of an ideal gas taken through a cycle AB...
Text Solution
|
- The figure below shows the variation of specific heat capacity (C) of ...
Text Solution
|
- A container of fixed volume has a mixture of a one mole of hydrogen an...
Text Solution
|
- In plotting stress versus strain curves for two materials P and Q, a s...
Text Solution
|
- An ideal monoatomic gas is confined in a horizontal cylinder by a spri...
Text Solution
|