A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SIMPLE HARMONIC MOTION
ALLEN |Exercise Exercise-05 [A]|39 VideosSIMPLE HARMONIC MOTION
ALLEN |Exercise Exercise-05 [B]|12 VideosSIMPLE HARMONIC MOTION
ALLEN |Exercise Exercise-04 [A]|20 VideosRACE
ALLEN |Exercise Basic Maths (Wave Motion & Dopplers Effect) (Stationary waves & doppler effect, beats)|25 VideosTEST PAPER
ALLEN |Exercise PHYSICS|4 Videos
Similar Questions
Explore conceptually related problems
ALLEN -SIMPLE HARMONIC MOTION-Exercise-04 [B]
- The wavelength involved in the spectrum of deuterium (.(1)^(2)D) are s...
Text Solution
|
- The manifestation of band structure in solids is due to
Text Solution
|
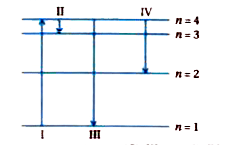
- The diagram shows the energy levels for an electron in a certain atom....
Text Solution
|
- Which of the following transitions in hydrogen atoms emit photons of h...
Text Solution
|
- Suppose an electron is attracted towards the origin by a force (k)/(r ...
Text Solution
|
- The transtion from the state n = 4 to n = 3 in a hydrogen like atom r...
Text Solution
|
- Energy required for the electron excitation in Li^(+2) from the first ...
Text Solution
|
- Hydrogen atom is excited from ground state to another state with princ...
Text Solution
|
- Dia-atomic molecule is composed of masses m(1) and m(2) and distance b...
Text Solution
|
- In a hydrogen like atom electron make transition from an energy level ...
Text Solution
|
- Hydrogen (""(1)H^(1)), Deuterium (""(1)H^(2)), single ionised Helium (...
Text Solution
|
- The radiation corresponding to 3 to 2 transition of hydrogen atom fall...
Text Solution
|
- Work function of sodium and copper is 2.3 eV and 4.5 eV respectively.R...
Text Solution
|
- Formation of covalent bonds in compounds exhibits …….nature.
Text Solution
|
- When frequencies f(1) and f(2) are incident on two indentical photo se...
Text Solution
|
- A radiation of energy 'E' falls normally on a perfectly reflecting sur...
Text Solution
|
- According to Einstein's photoelectric equation graph of kinetic energy...
Text Solution
|
- The work function of a metal surface is 4.2 eV. The maximum wavelength...
Text Solution
|
- A photocell is placed at 1 m distance from light source when distance ...
Text Solution
|
- If kinetic energy of emitted electron is made double then its de-Brogl...
Text Solution
|