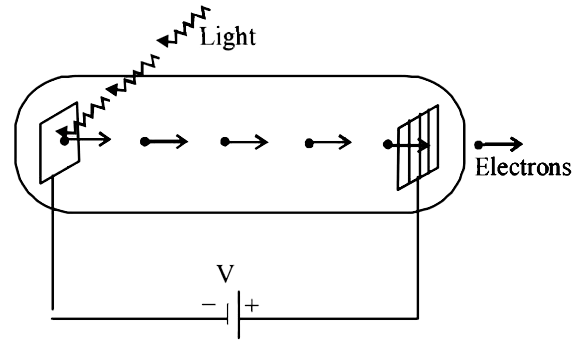
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SIMPLE HARMONIC MOTION
ALLEN |Exercise MCQ s|8 VideosSIMPLE HARMONIC MOTION
ALLEN |Exercise Match The Column|9 VideosSIMPLE HARMONIC MOTION
ALLEN |Exercise Exercise - 04[A]|25 VideosRACE
ALLEN |Exercise Basic Maths (Wave Motion & Dopplers Effect) (Stationary waves & doppler effect, beats)|25 VideosTEST PAPER
ALLEN |Exercise PHYSICS|4 Videos
Similar Questions
Explore conceptually related problems
ALLEN -SIMPLE HARMONIC MOTION-Exercise-05(B)
- The fig. shows the variation of photon current with anode potential fo...
Text Solution
|
- A photon and an electron have equal energy E . lambda("photon")//lambd...
Text Solution
|
- After 280 days, the activity of a radioactive sample is 6000 dps. The ...
Text Solution
|
- A photon collides with a stationary hydrogen atom in ground state inel...
Text Solution
|
- Kalpha wavelength emitted by an atom of atomic number Z=11 is lambda. ...
Text Solution
|
- If a star can convert all the He nuclei completely into oxygen nuclei....
Text Solution
|
- Half-life of a radioactive substance A is 4 days. The probability that...
Text Solution
|
- The largest wavelength in the ultraviolet region of the hydrogen spect...
Text Solution
|
- Electrons with de-Broglie wavelength lambda fall on the target in an X...
Text Solution
|
- In the options given below, let E denote the rest mass energy of a nuc...
Text Solution
|
- Which one of the following statement is WRONG in the context of X- ray...
Text Solution
|
- A radioactive sample S1 having an activity of 5muCi has twice the numb...
Text Solution
|
- Photoelectric effect experiments are performed using three different m...
Text Solution
|
- A pulse of light of duration 100 ps is absorbed completely by a small ...
Text Solution
|
- If lambda(Cu) is the wavelength of K(alpha) X-ray line of copper (atom...
Text Solution
|
- A metal is illumimated by light of two different wavelength 248nm and ...
Text Solution
|
- In a historical experiment to determine Planck's constant, a metal sur...
Text Solution
|
- The electrostatic energy of Z protons uniformly distributed throughout...
Text Solution
|
- As accident in a nuclear laboratory resulting in deposition of a certa...
Text Solution
|
- Light of wavelength lambda(ph)falls on a cathode plate inside a vacuum...
Text Solution
|