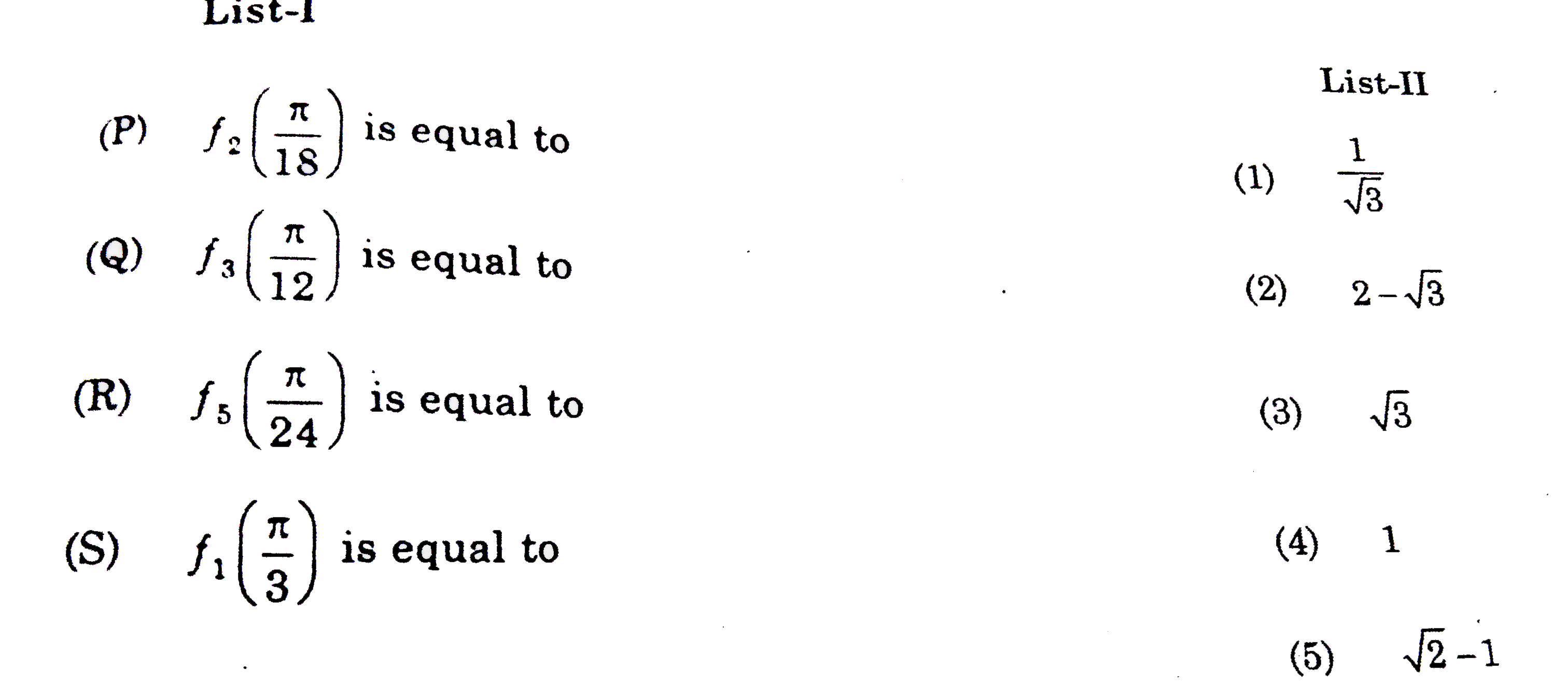A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN -TEST PAPER-MATHS
- Consider the equation x^2+p|x|+1=0, where p is real coefficient Matc...
Text Solution
|
- Let f(x)=(x^2-11x+18)/(x^2-7x+12) Match List-I with List-II and sele...
Text Solution
|
- fn(x)=(underset(r=1)overset(n)Sigmasin(rx))/(underset(r=1)overset(n)Si...
Text Solution
|
- If 2p=x+1/x and 2q=y+1/y, then the value of ((pq+sqrt((p^2-1)(q^2-1)))...
Text Solution
|
- Let f(x)=(x^2-ax-2)/(x^2+x+1) such that -3lt f(x)lt 2AA x in R and com...
Text Solution
|
- Let P be minimum value of 9sin^2x+16cosec^2x and S be the minimum valu...
Text Solution
|
- If the range of real values of k for which equation 2"log"3^2x-|log3x|...
Text Solution
|
- There are three units circles each of which is tangential to the other...
Text Solution
|
- If x and y are positive real numbers such that x^3+8y^3+24xy=64 , then...
Text Solution
|
- Total maximum number of degenerated orbitals in n=3 shell in H-atom ?
Text Solution
|
- Ground state electronic configuration of 'C' written as [He] 2s^2 2p^2...
Text Solution
|
- Maximum number of electrons in a subshell can be calculated by followi...
Text Solution
|
- Select correct set of quantum numbers ?
Text Solution
|
- Which of the following species have highest magnetic moment (by using ...
Text Solution
|
- Find the total number of exchange in d^8 configuration ?
Text Solution
|
- Which of the following oxide is most acidic ?
Text Solution
|
- Which of the following is correct order of acidic strength ?
Text Solution
|
- Select the INCORRECT order of atomic size ?
Text Solution
|
- Select the CORRECT order of ionisation energy ?
Text Solution
|
- Which of the following is endothermic process ?
Text Solution
|