Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN -TEST PAPERS-PHYSICS
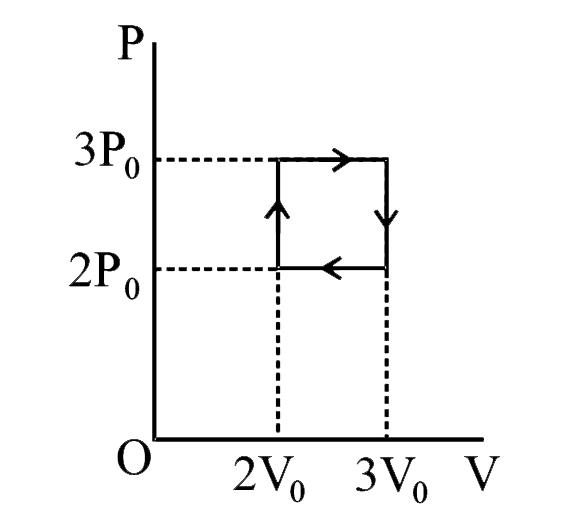
- A thermodynamic system is taken from an initial state X along the path...
Text Solution
|
- A thermodynamic system is taken from an initial state X along the path...
Text Solution
|
- The below P-V diagram represents the thermodynamic cycle for one mole ...
Text Solution
|
- A container with insulating walls is divided into two equal parts by a...
Text Solution
|
- If C(P) and C(V) denote the specific heats of nitrogen per unit gram a...
Text Solution
|
- A new monatomic ideal gas is discovered and named Wellsium. A pure 4-m...
Text Solution
|
- A sample of gas is caused to go through the cycle shown in the PV diag...
Text Solution
|
- 0.02 moles of an ideal gas undergo a cycle consisting of an isochoric ...
Text Solution
|
- A part of circuit is shown in figure. Choose the CORRECT option(s):
Text Solution
|
- A binary star system consists of two stars having masses M and 2M. Th...
Text Solution
|
- Three electric lamps designed for a voltage of 110V each are rated at ...
Text Solution
|
- Three metallic plates out of which middle is given charge Q as shown i...
Text Solution
|
- A neutral conductor of radius R has a spherical cavity of radius R/4 a...
Text Solution
|
- An ellipsoidal gaussion surface with semi major axis a and minor axis ...
Text Solution
|
- For given circuit, select correct alternative(s)
Text Solution
|
- If Q is the charge on the plates of a capacitor of capacitance C,V the...
Text Solution
|
- Case I- represents two concentric spheres of inner radius a and outer ...
Text Solution
|
- Case I- represents two concentric spheres of inner radius a and outer ...
Text Solution
|
- Five identical capacitors (initially uncharged) are connected in circu...
Text Solution
|
- Five identical capacitors (initially uncharged) are connected in circu...
Text Solution
|