A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN -TEST PAPERS-PHYSICS
- Three metal rods A,B and C of same length and cross-section are placed...
Text Solution
|
- A diatomic gas is kept in a closed container of constant volume. Due t...
Text Solution
|
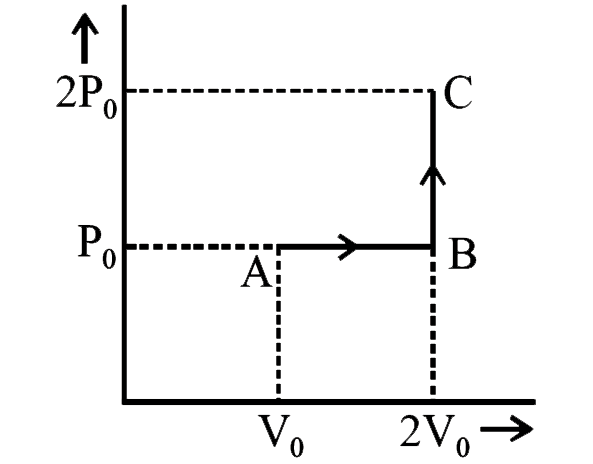
- One mole of an ideal monoatomic gas is taken from A to C along the pat...
Text Solution
|
- A particle moves such that its position x varies with time according t...
Text Solution
|
- For a particle moving on x-axis velocity time graph is shown in figure...
Text Solution
|
- A long playing records revolves with a speed of 33 1/3 rev/min and ha...
Text Solution
|
- A block of mass 10kg is at rest on a frictionless wedge. Angle of incl...
Text Solution
|
- Figure shows position versus time graph of two rabbits running opposit...
Text Solution
|
- Two boats A and B starts from A and B respectively and both boats reac...
Text Solution
|
- Two boats A and B starts from A and B respectively and both boats reac...
Text Solution
|
- In a small pan of heat capacity 300J//.^(@)C we pour 0.5 litre water a...
Text Solution
|
- In a small pan of heat capacity 300J//.^(@)C we pour 0.5 litre water a...
Text Solution
|
- Compound A(2)B crystallise in hexagonal lattice at low temperature. Un...
Text Solution
|
- Given: C(2)H(2)(g)+2H(2)(g)toC(2)H(6)(g) At 400K it was observed du...
Text Solution
|
- Whch of the following statement in INCORRCT about CO(3)^(2-) ion?
Text Solution
|
- Bond energy is maximum for
Text Solution
|
- Consider following structures and select INCORRECT order.
Text Solution
|
- Which of the following pairs are resonance structures of each other?
Text Solution
|
- Identify compound incorrectly match with total number of Deuterium exc...
Text Solution
|
- Which is/are correct statements about a solution containing CH(3)COCH(...
Text Solution
|