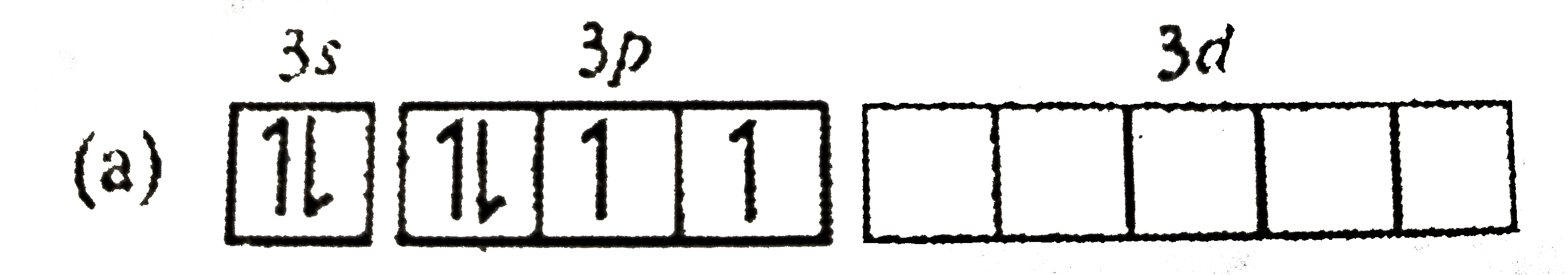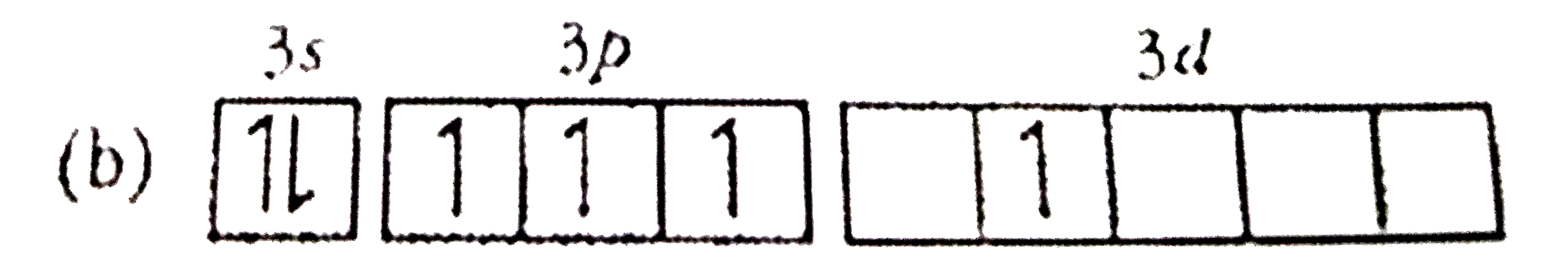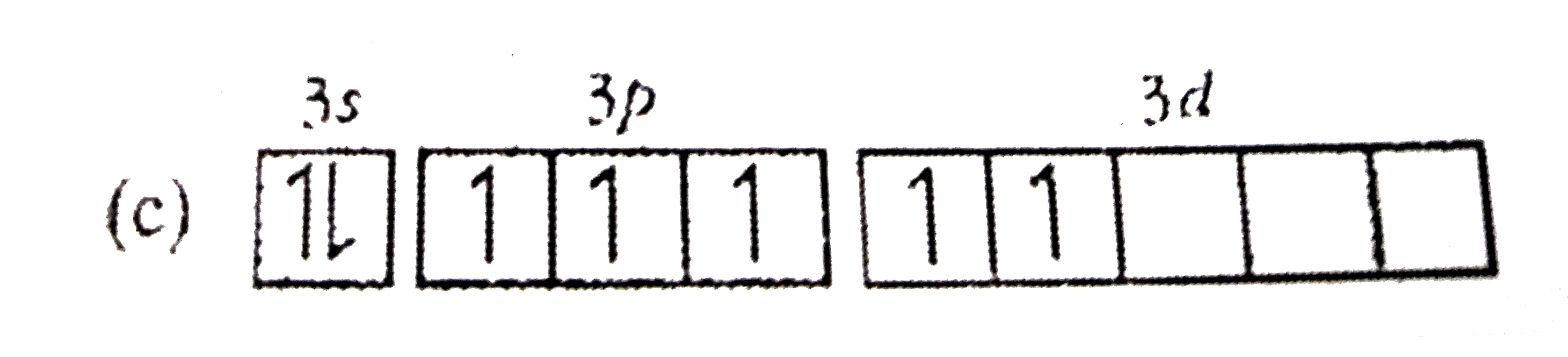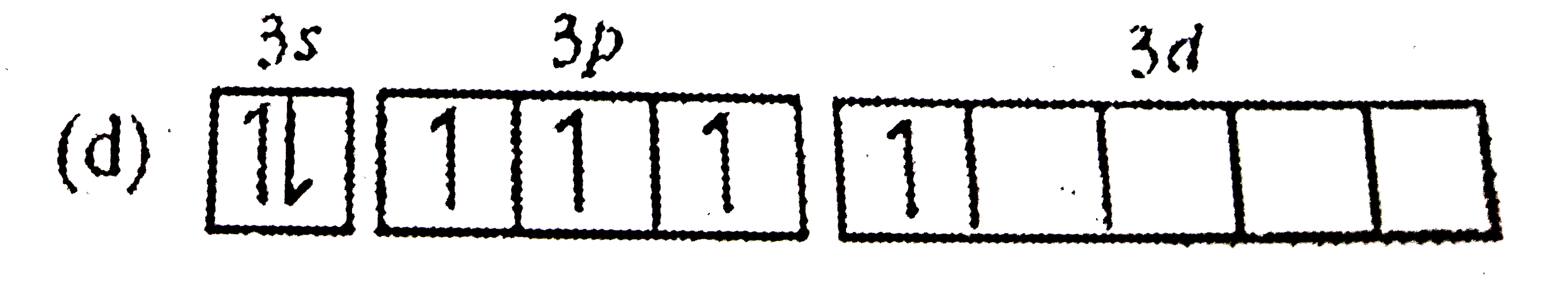A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following has maximum energy ?
Text Solution
|
- Which of the following has maximum hydration energy ?
Text Solution
|
- Which of the following has maximum hydrogenation energy.
Text Solution
|
- Which of the following has maximum bond energy?
Text Solution
|
- निम्न में से किसकी बन्ध ऊर्जा अधिकतम है ?
Text Solution
|
- Which of the following conformation has maximum energy ?
Text Solution
|
- Which one of the following has the maximum energy ?
Text Solution
|
- Which of the following has maximum bond energy
Text Solution
|
- निम्न में से किस बंध की ऊर्जा अधिकतम है
Text Solution
|