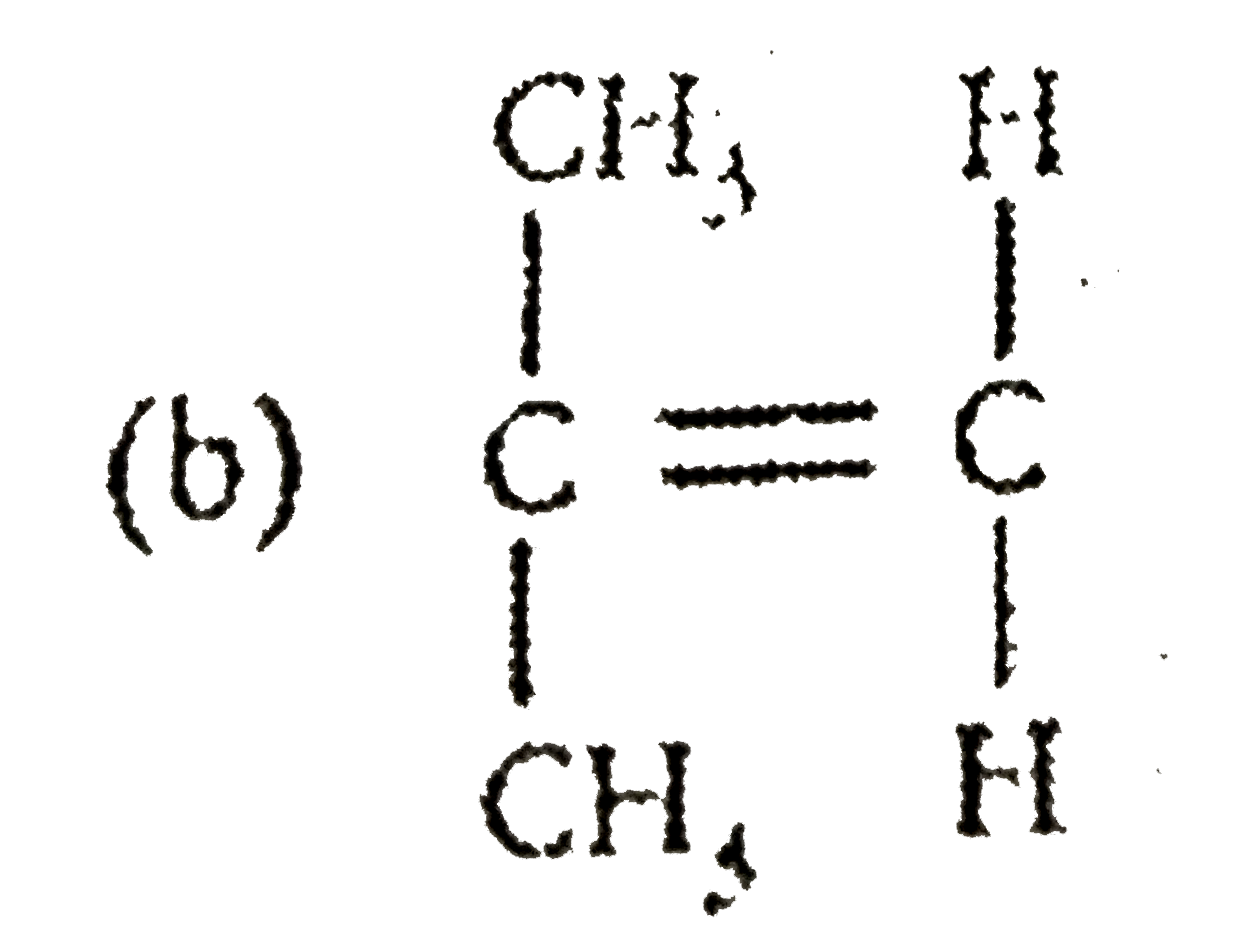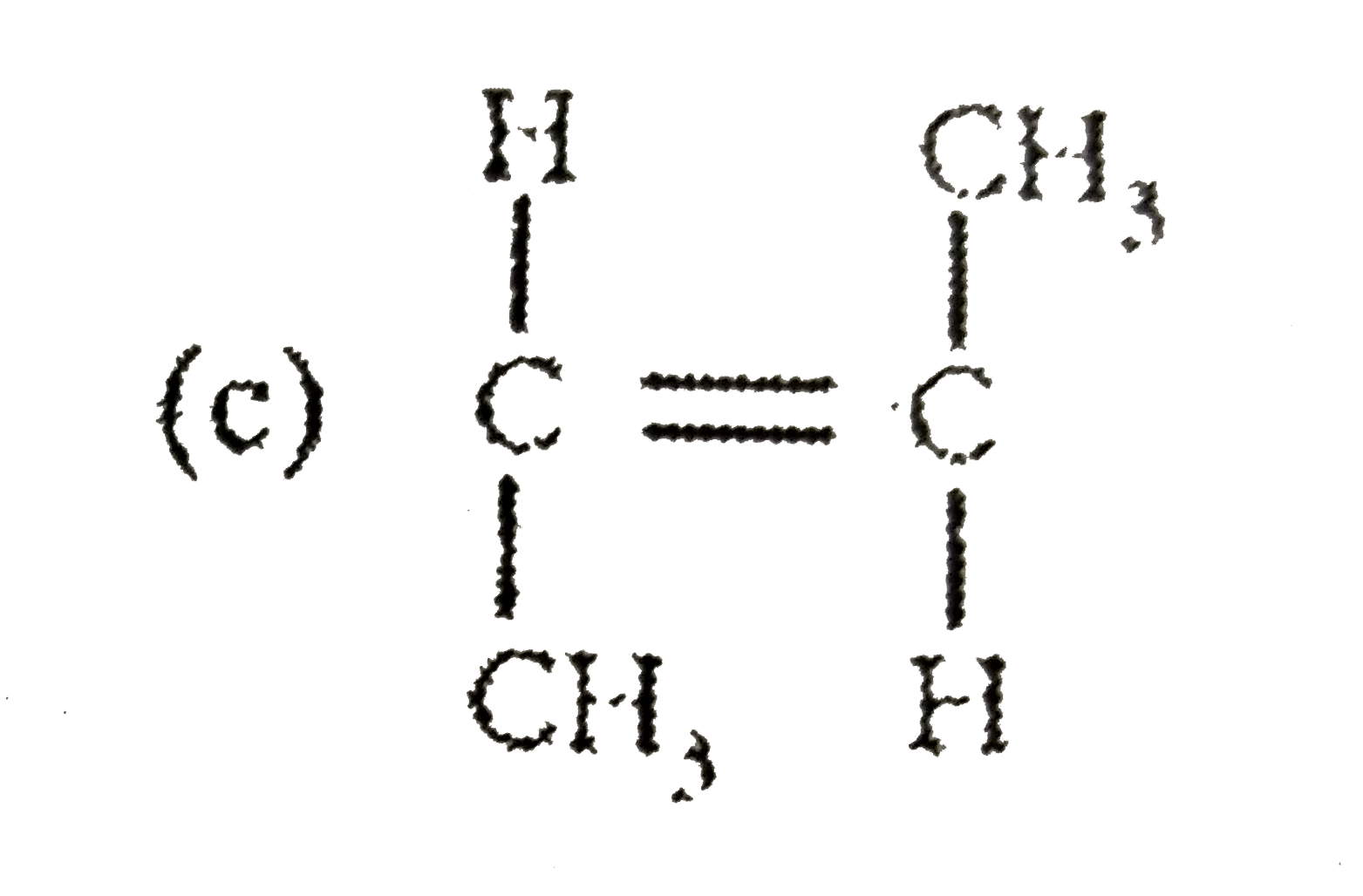To determine which of the given compounds has the highest dipole moment, we need to analyze the molecular structures and the electronegativities of the atoms involved. Let's go through the options step by step.
### Step 1: Identify the Compounds
The question provides four options. Let's denote them as follows:
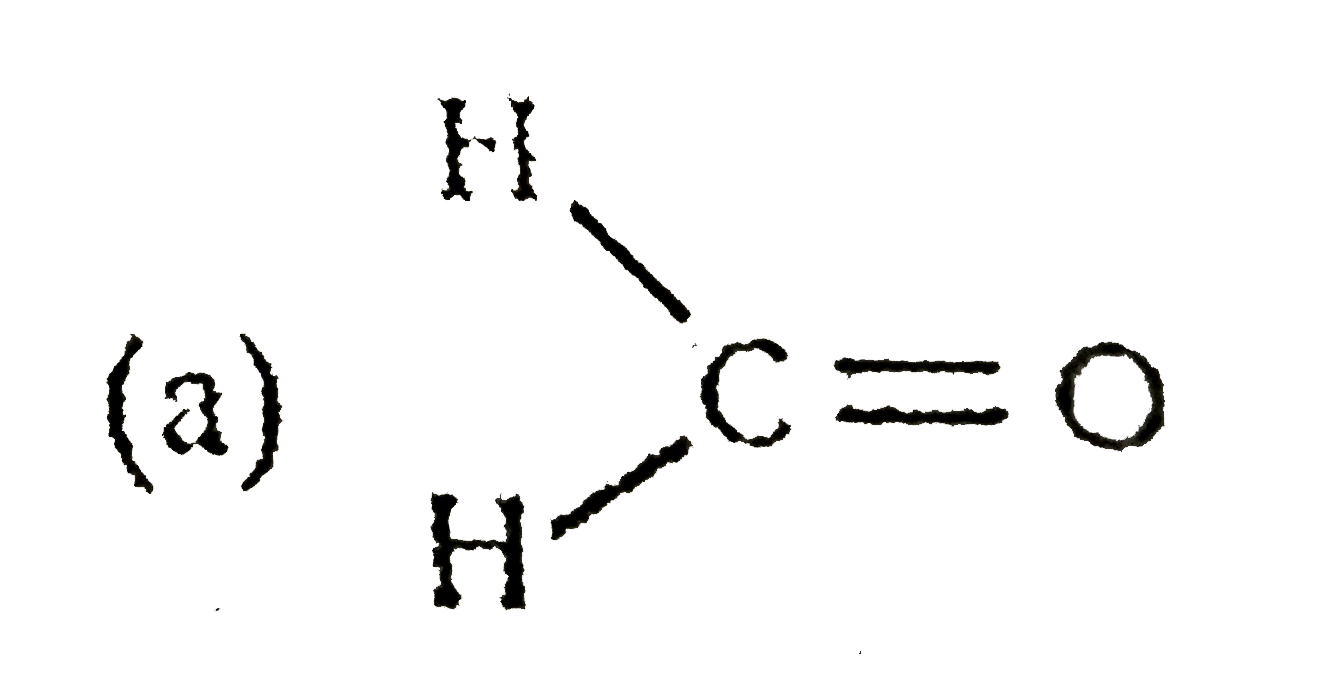
1. **Option A**: Carbon-Hydrogen-Hydrogen double bond-Oxygen
2. **Option B**: Carbon double bond-Carbon, Hydrogen, Hydrogen
3. **Option C**: Carbon double bond-Carbon, CH3, Hydrogen, CH3, Hydrogen
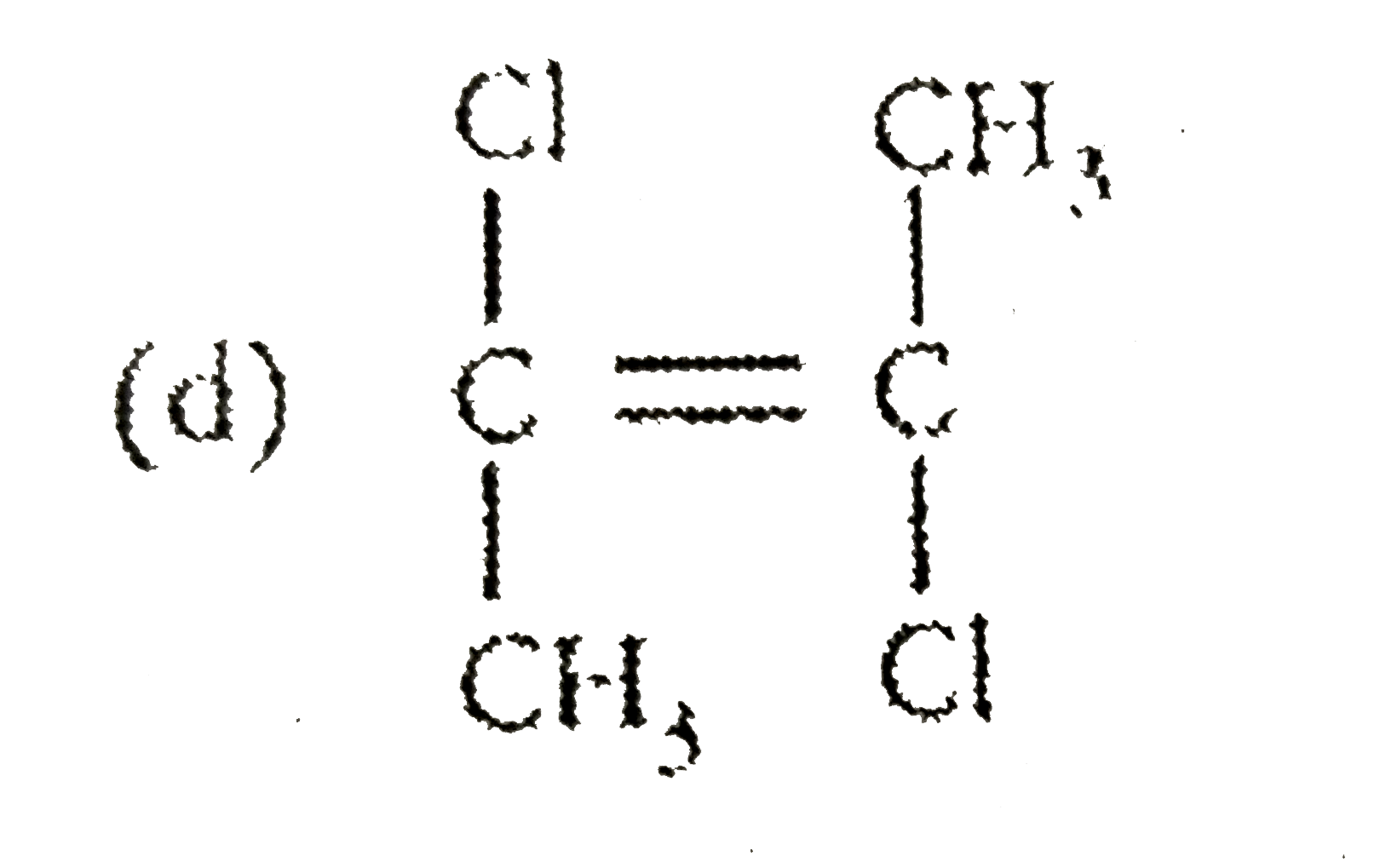
4. **Option D**: Carbon double bond-Carbon, Cl, Cl, CH3, CH3, CH3, CH3
### Step 2: Determine Electronegativity
- **Oxygen (O)** is highly electronegative compared to Carbon (C) and Hydrogen (H).
- **Chlorine (Cl)** is also electronegative but less than Oxygen.
### Step 3: Analyze Each Option
1. **Option A**: The presence of a double bond with Oxygen suggests a significant dipole moment due to the high electronegativity of Oxygen. The dipole moment will point towards the Oxygen atom.
2. **Option B**: This compound has a symmetrical structure (C=C) with two Hydrogens on each Carbon. The dipole moments will cancel out, resulting in a net dipole moment of zero.
3. **Option C**: Similar to Option B, the structure is symmetrical with CH3 groups on either side of the double bond. The dipole moments will also cancel out, leading to a net dipole moment of zero.
4. **Option D**: This compound has two Chlorine atoms, which are electronegative. However, the symmetry of the molecule will cause the dipole moments to cancel out, resulting in a net dipole moment of zero.
### Step 4: Conclusion
From the analysis, Option A has the highest dipole moment due to the presence of the highly electronegative Oxygen atom, which creates a significant polarity in the molecule. The other options either have symmetrical structures or less electronegative atoms, leading to lower or zero dipole moments.
### Final Answer
**Option A** has the highest dipole moment.