A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
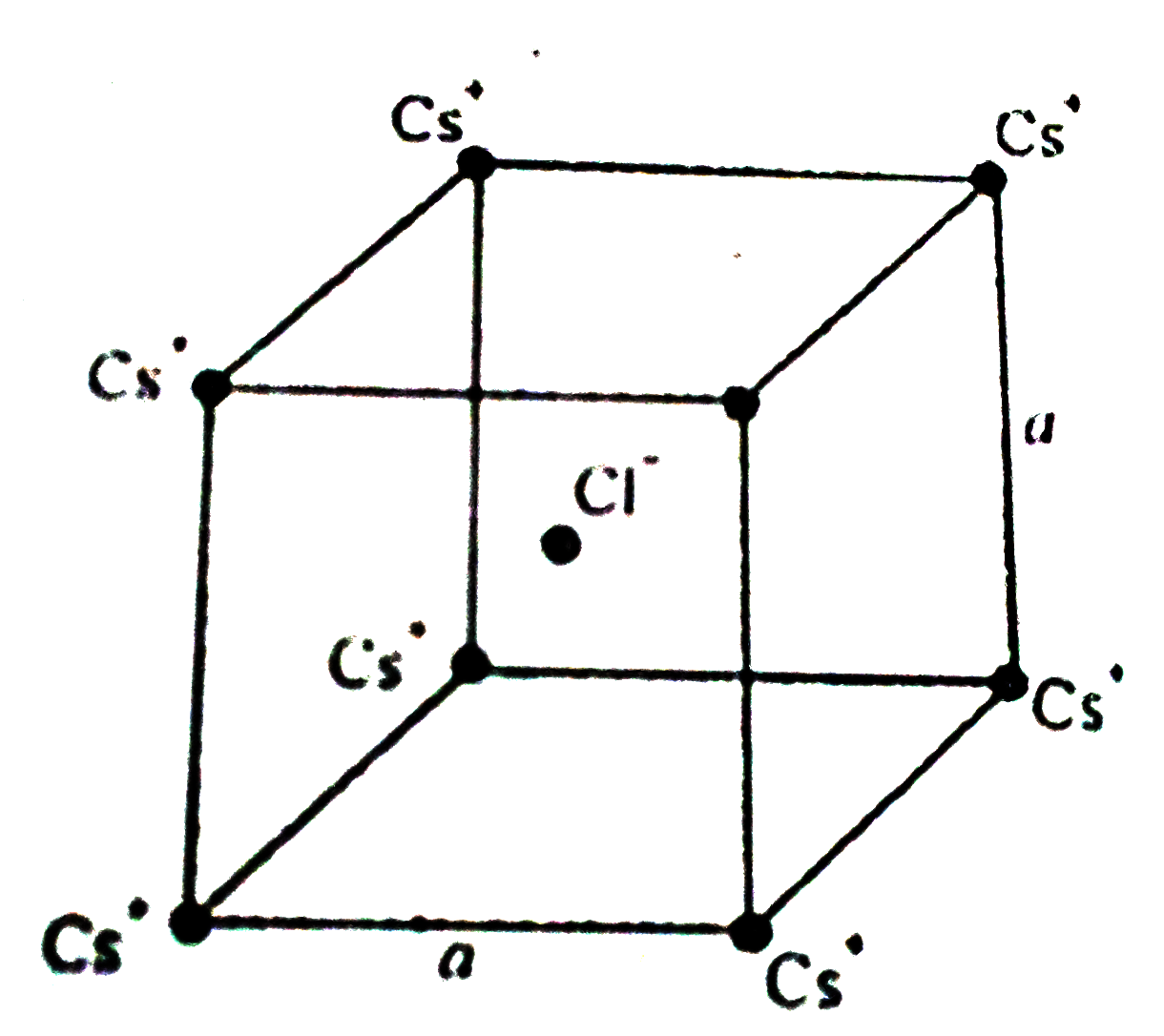
- In the basic CsCI crystal structure,Cs^(+) andCI^(-) ions are arrnged ...
Text Solution
|
- In CsCI lattice the coordination number of Cs ion is
Text Solution
|
- CsCl has bcc structure with Cs^(+) at the centre and Cl^(-) ion at eac...
Text Solution
|
- In the cubic crystal of CsCl (d = 3.97 g" cm "^(-3) ) the eight corner...
Text Solution
|
- In the basic CsCI crystal structure, Cs^(+) and CI^(-) ions are arrnge...
Text Solution
|
- CsCI में Cs^+ आयन 6 Cl^- आयनों से घिरा रहता है।
Text Solution
|
- In a cubic crystal of CsCl (density = 3.97 gm//cm^(3)) the eight corne...
Text Solution
|
- In a cubic crystal of CsCl (density = 3.97 gm/ cm^(3) ) the eight corn...
Text Solution
|
- In the basic CsCl crystal structure Cs^(+) And Cl^(-) The ions lie in ...
Text Solution
|