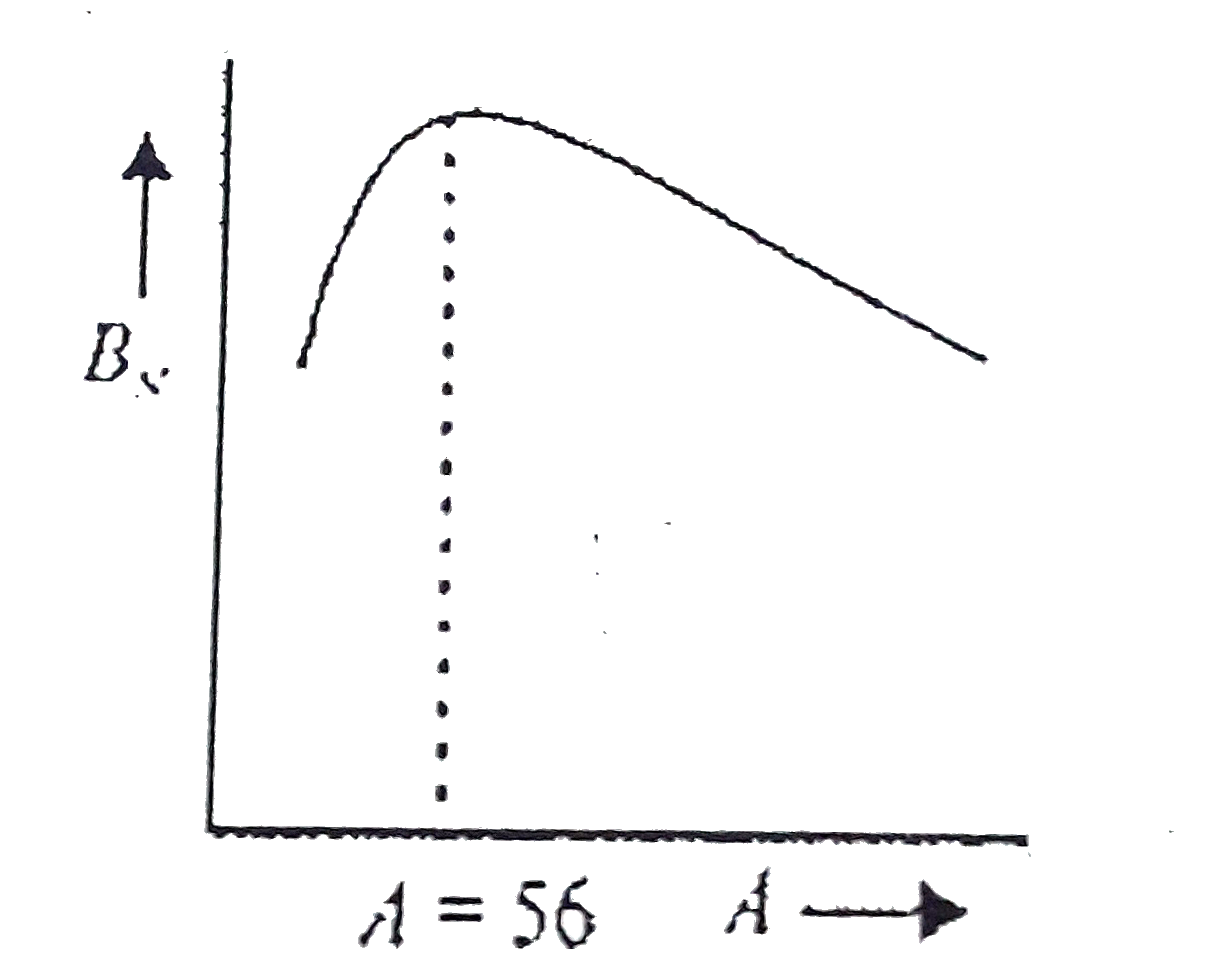
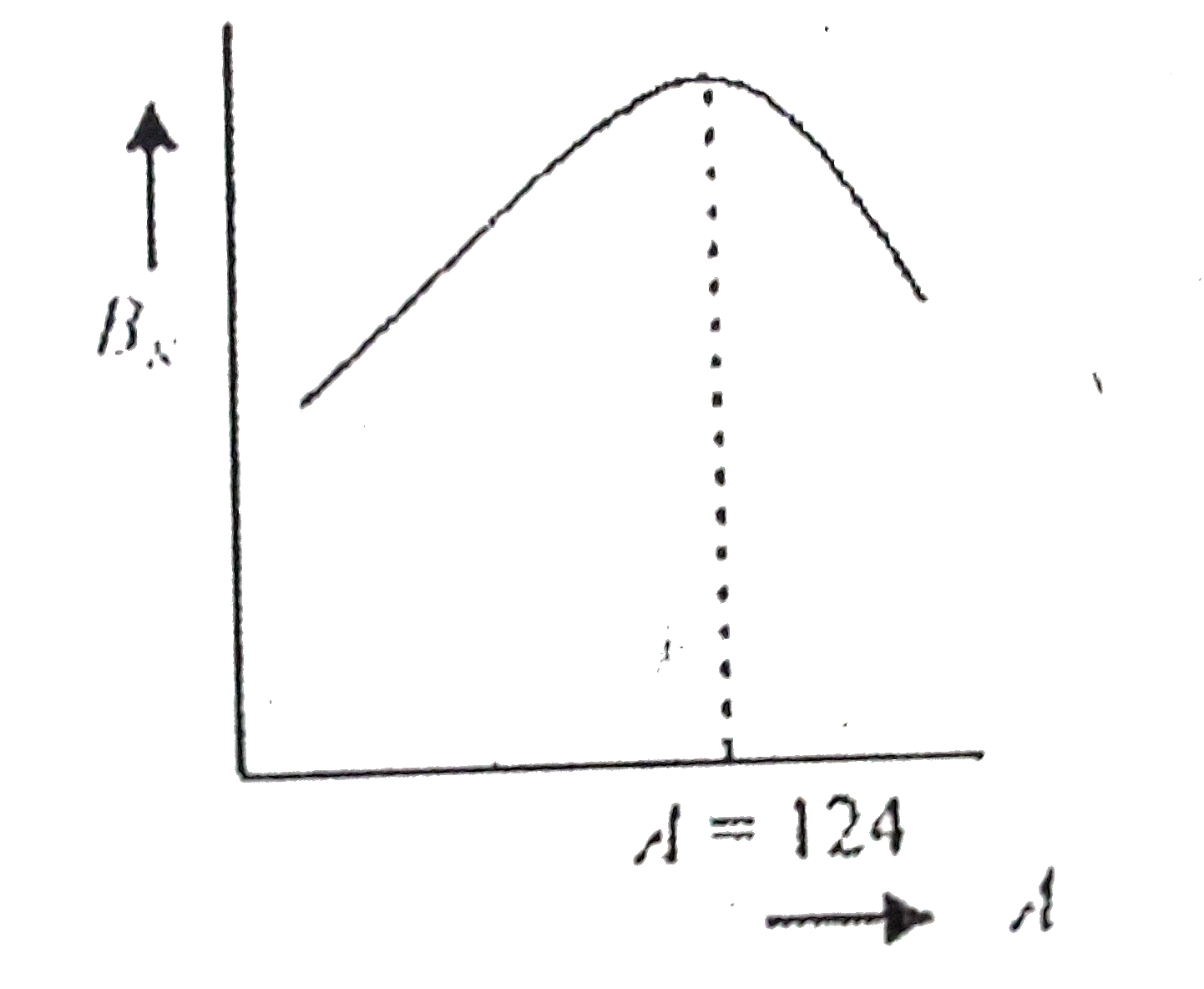
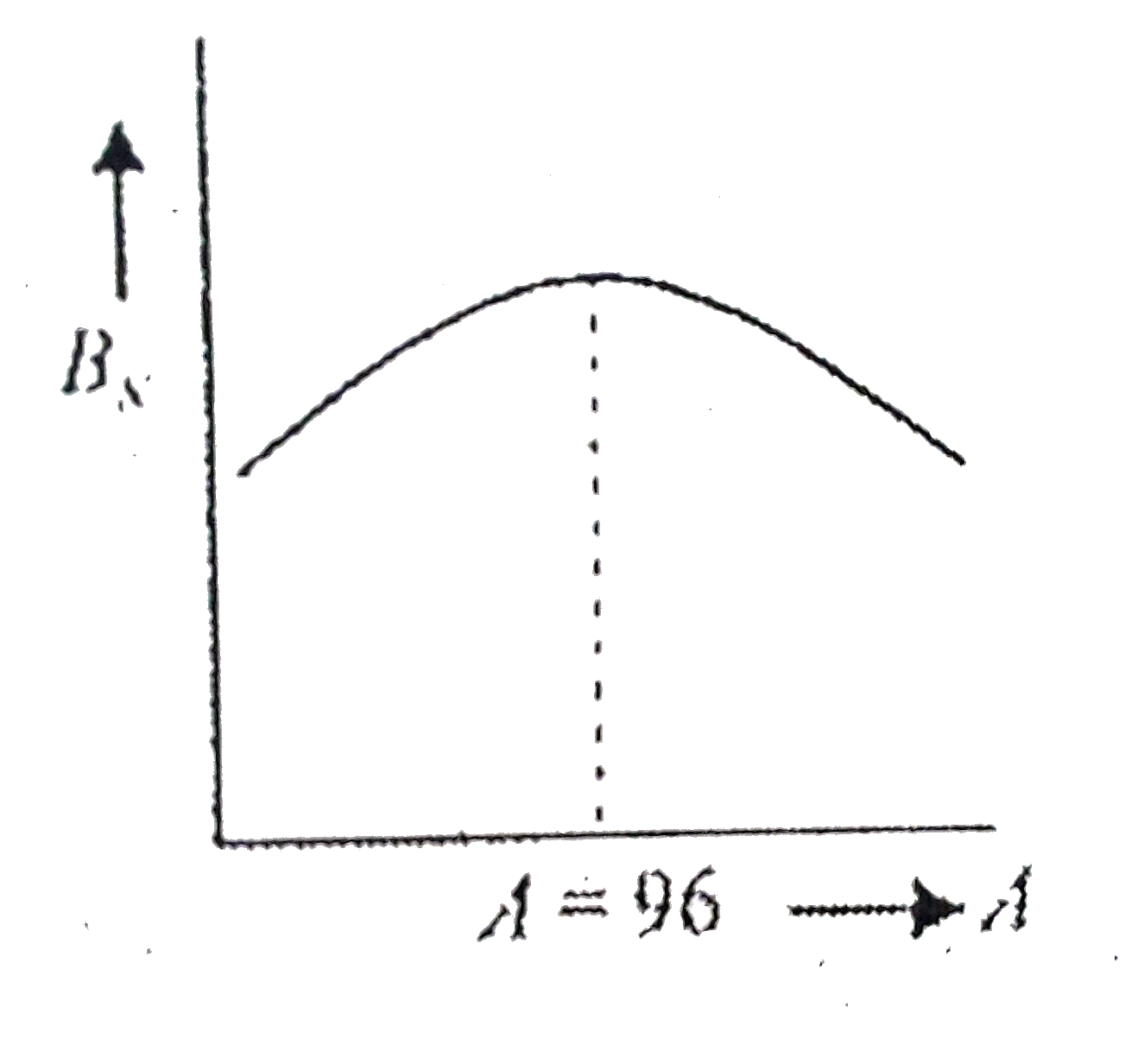
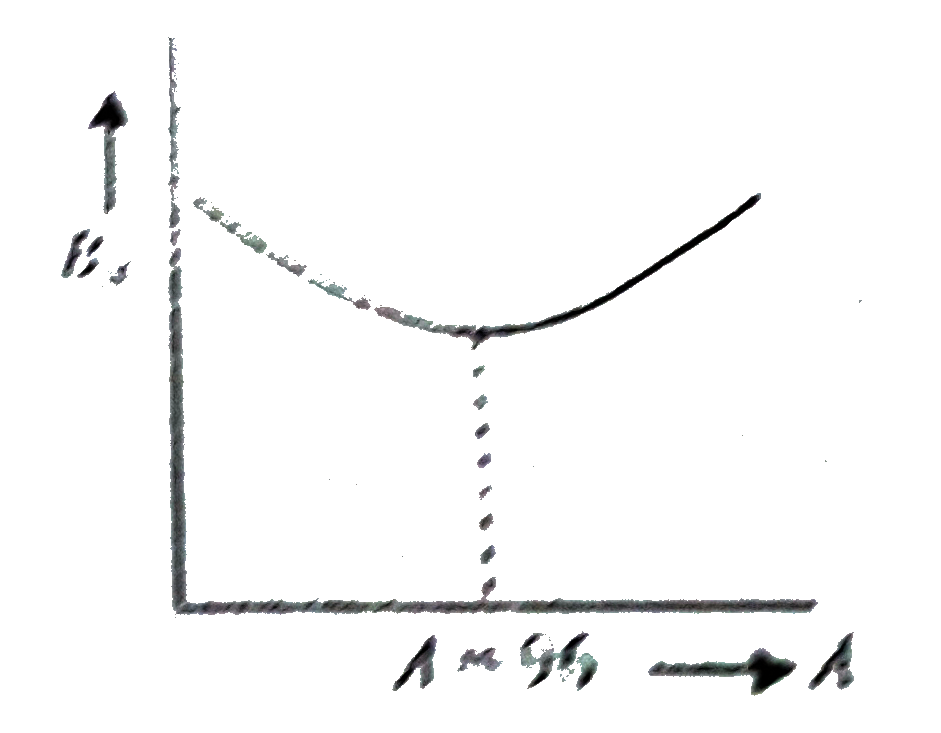
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The dependence of binding energy per nucleon, BN on the mass number, A...
Text Solution
|
- Variation Of Binding Energy Of Per Nucleon With Mass Number
Text Solution
|
- The dependence of binding energy per nucleon, BN on the mass number, A...
Text Solution
|
- Draw the graph showing thervariation of binding energy per nucleon wit...
Text Solution
|
- Binding energy per nucleon relation with mass number
Text Solution
|
- As the mass number increase binding energy per nucleon :
Text Solution
|
- किसी नाभिक में प्रति न्यूक्लिऑन बंधन ऊर्जा दर्शाती है
Text Solution
|
- What is Mass defect, binding energy and binding energy per nucleon?
Text Solution
|
- The dependence of binding energy per nucleon, BN on the mass number, A...
Text Solution
|
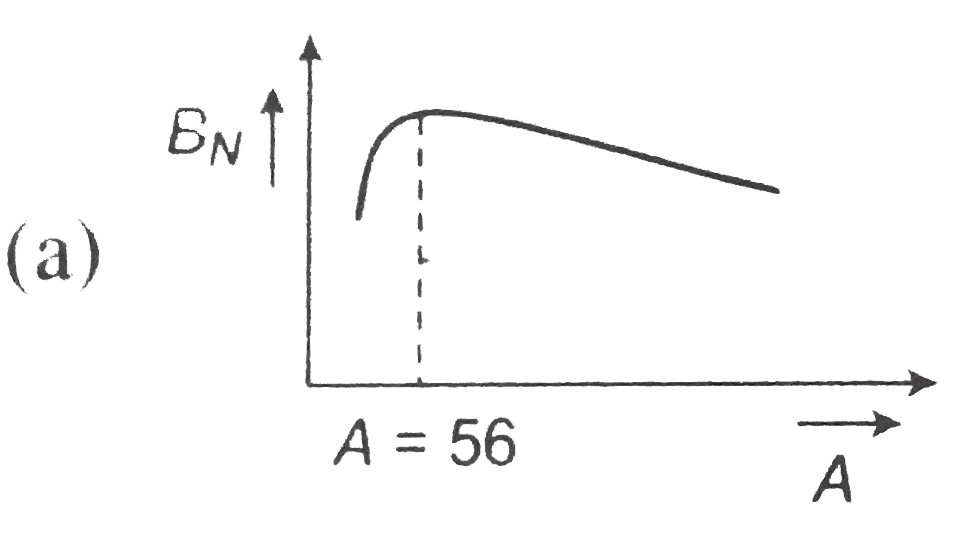 , (b)
, (b) 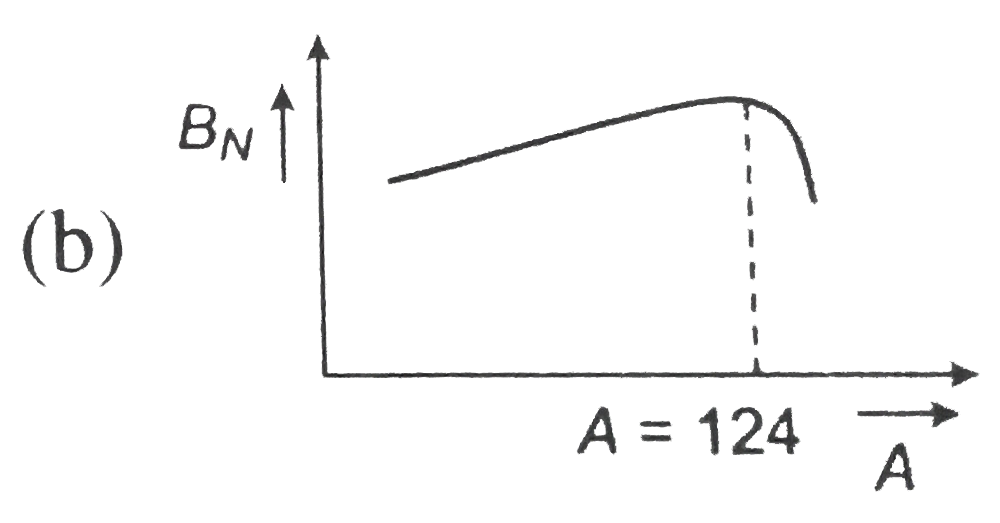 ,
, 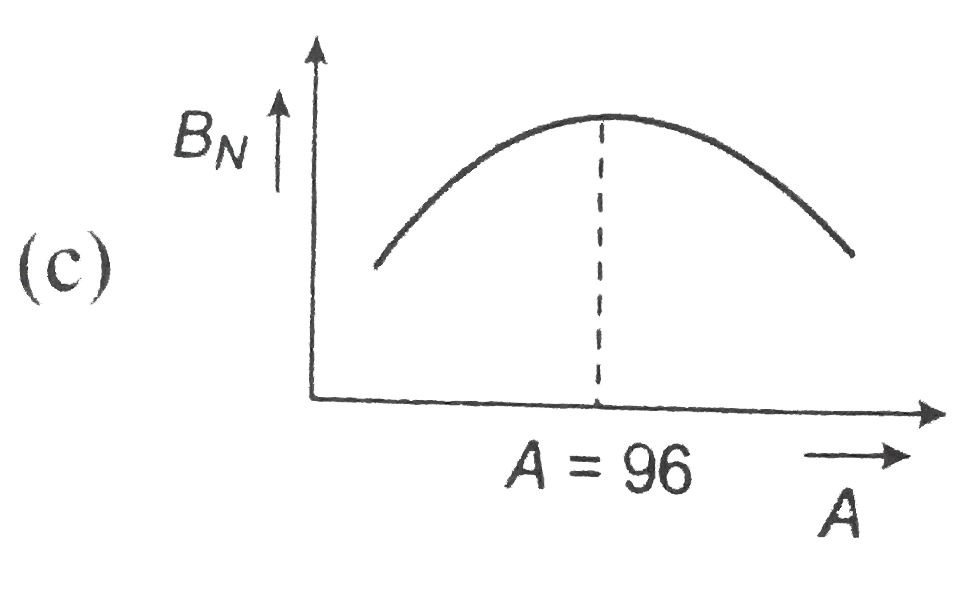 , (d)
, (d) 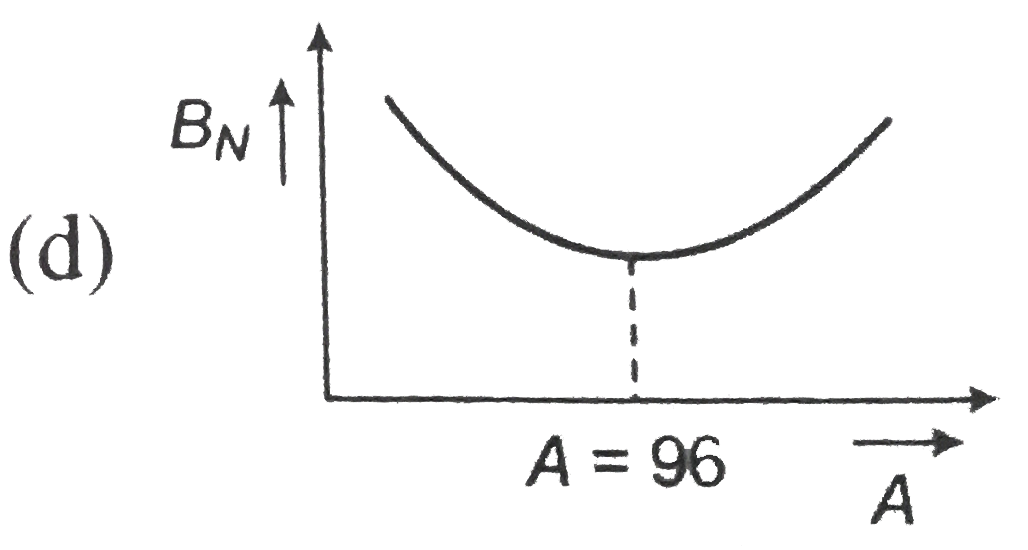 .
.