A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is acidic in nature ?
Text Solution
|
- Which of the following is acidic in nature ?
Text Solution
|
- Which of the following is acidic in nature ?
Text Solution
|
- Which of the following is acidic in nature ?
Text Solution
|
- Which of the following is acidic in nature-
Text Solution
|
- निम्नलिखित में से कौन सा अम्लीय प्रकृति का है |
Text Solution
|
- निम्नलिखित में से कौन सा अम्लीय प्रकृति का है |
Text Solution
|
- Which of the following is acidic in nature ?
Text Solution
|
- निम्नलिखित में से कौन प्रकृति में केवल अम्लीय है
Text Solution
|
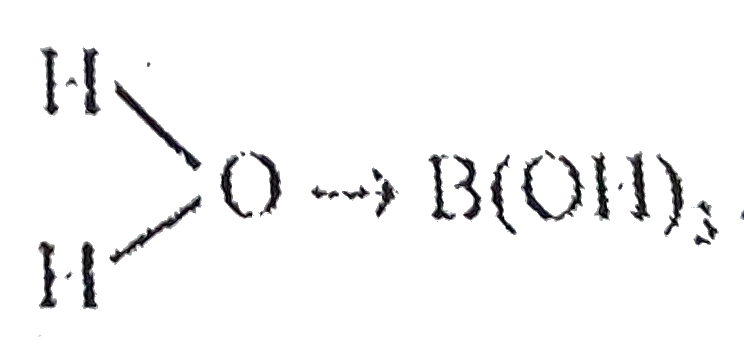 .In this species,`B^(3+)` ion,because of its small size,exercises a high polarizing power thereby pulling the sigma electeron charge of the coordinated O atom towards itself.The coordinated oxygen,in turn,pulls the sigma electron charge of the OH bond of the attached `H_(2)O` molecule towards itself.This facilitates the removal of `H^(+)` ion from the O-H bonds,as shown below
.In this species,`B^(3+)` ion,because of its small size,exercises a high polarizing power thereby pulling the sigma electeron charge of the coordinated O atom towards itself.The coordinated oxygen,in turn,pulls the sigma electron charge of the OH bond of the attached `H_(2)O` molecule towards itself.This facilitates the removal of `H^(+)` ion from the O-H bonds,as shown below