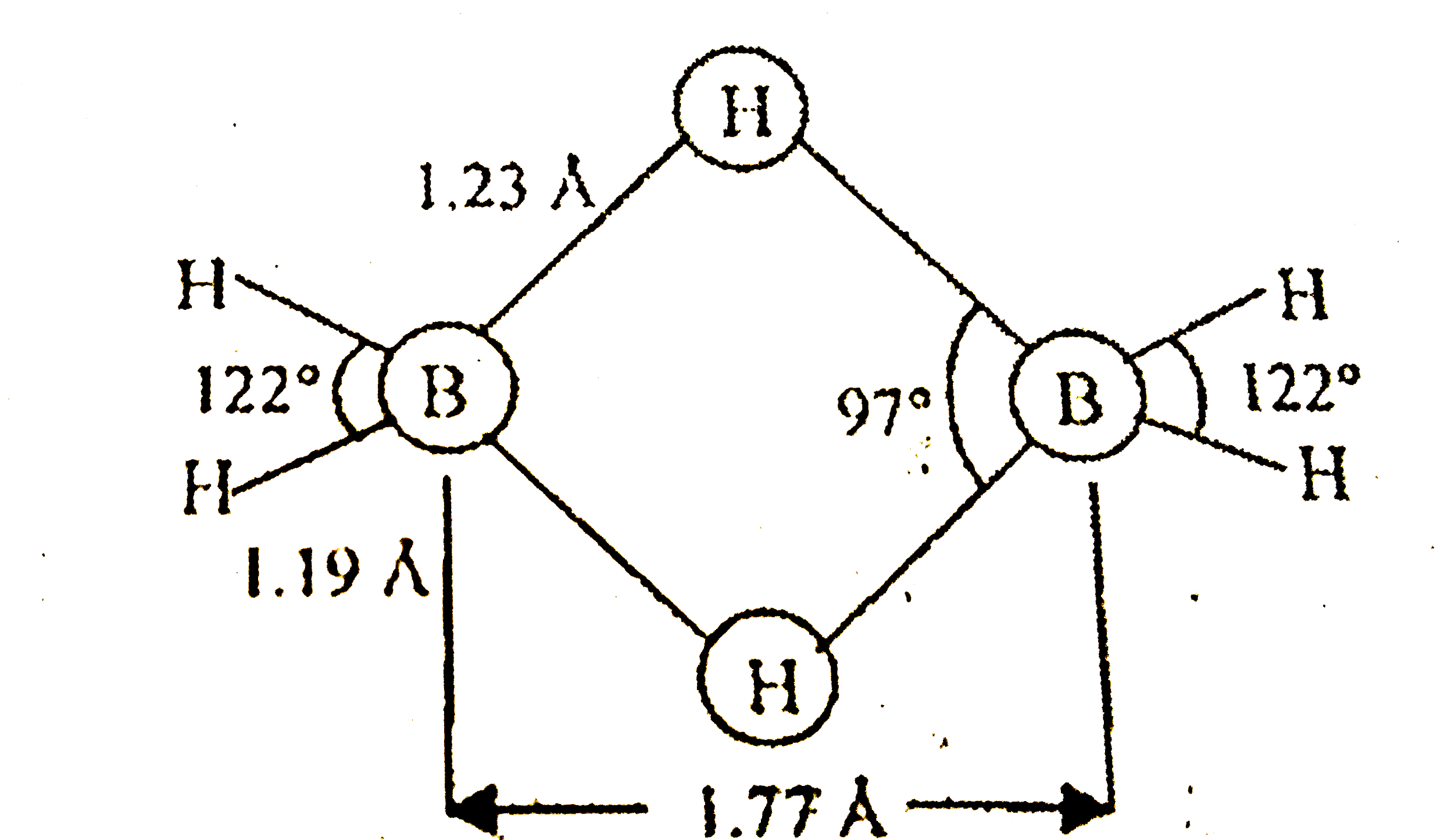
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In diborane the two H-B-H angles are nearly:
Text Solution
|
- In diborane, the two H-B-H angles are nearly
Text Solution
|
- There are two H-bridge bonds in diborane molecule because there are
Text Solution
|
- In diborane, the two H-B-H angles are nearly
Text Solution
|
- In diborane the two H-B-H angles are nearly
Text Solution
|
- In diborane, the H-B-H bond angle is 120^(@). The hybridization of bor...
Text Solution
|
- Diborane has ……………….. B-H bonds.
Text Solution
|
- The H-B-H bridged angle in diborane is
Text Solution
|
- The H-B-H bridged angle in diborane is
Text Solution
|