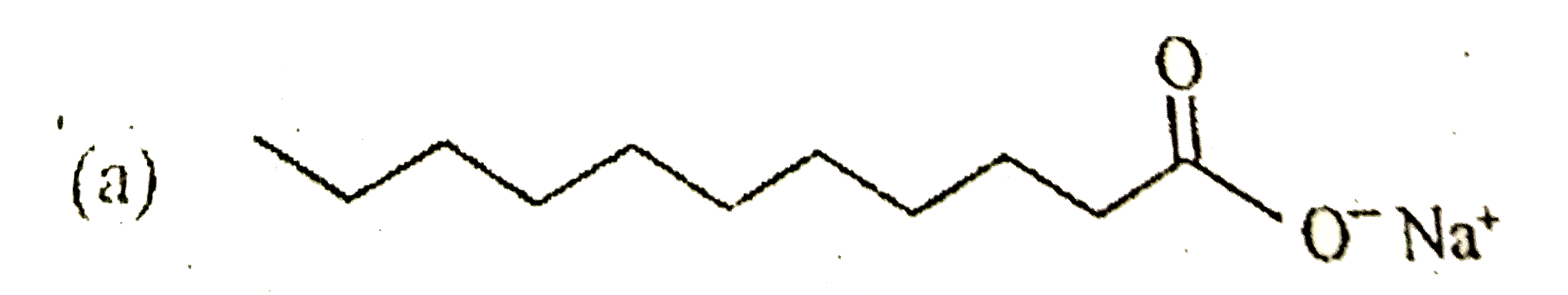
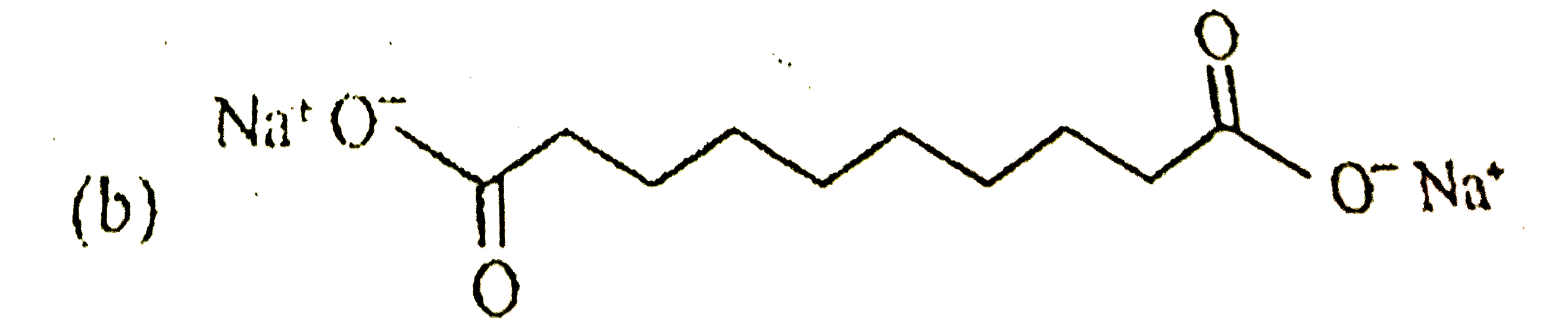
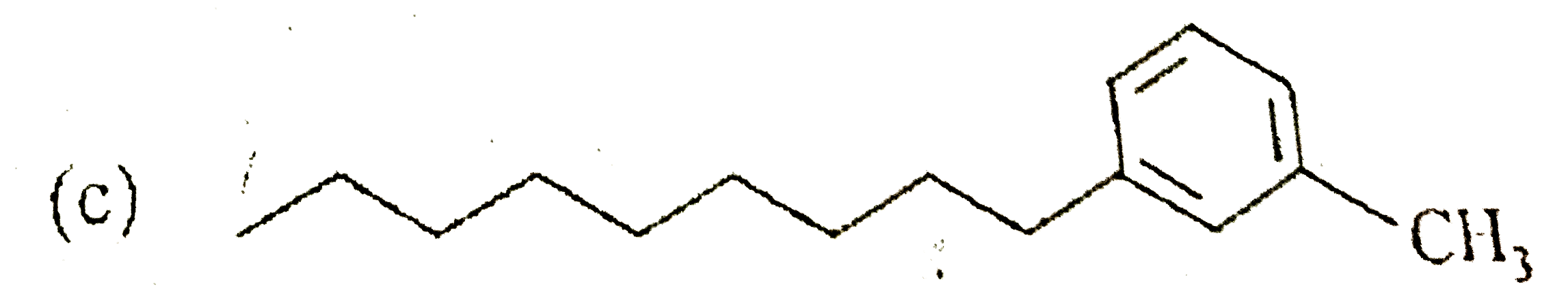
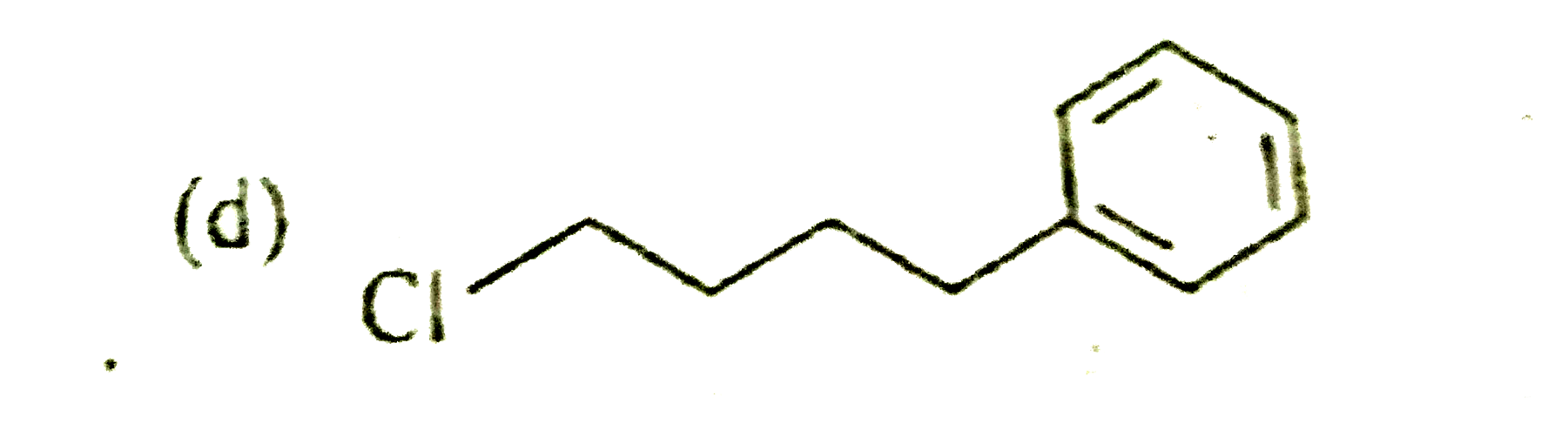
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following molecules is most suitable to disperse benzene ...
Text Solution
|
- Which of the following molecules is most suitable to disperse benzen i...
Text Solution
|
- Which of the following can disperse benzene in water
Text Solution
|
- Which of the following is the most suitable test to identify water
Text Solution
|
- Which of the following can disperse benzene in water?
Text Solution
|
- Which of the following molecules is most suitable to disperse benzene ...
Text Solution
|
- Which of the following is the most suitable method for removing the tr...
Text Solution
|
- Which of the following can disperse benzene in water?
Text Solution
|
- Which one of the following reactions is most suitable for the preparat...
Text Solution
|