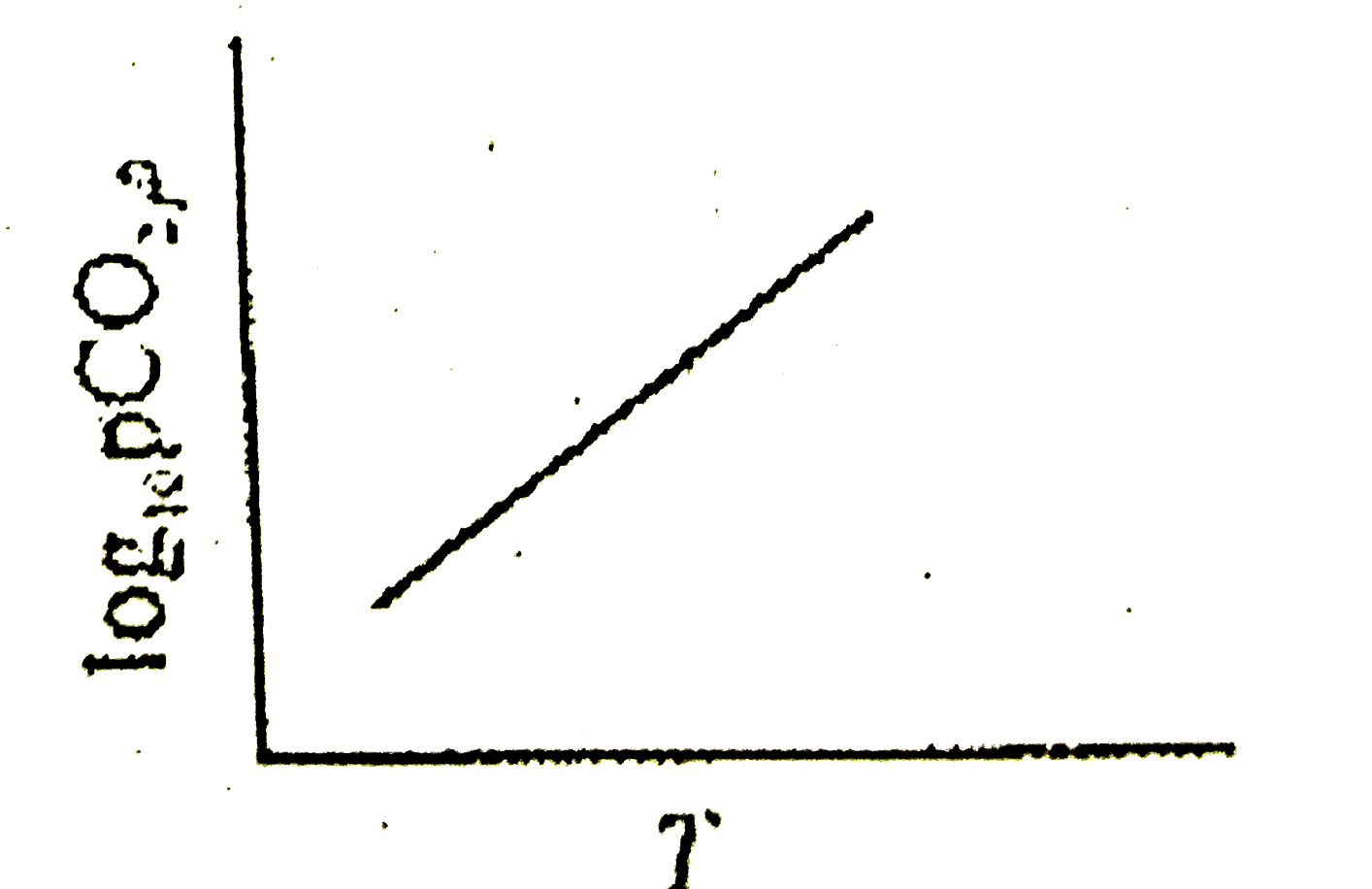
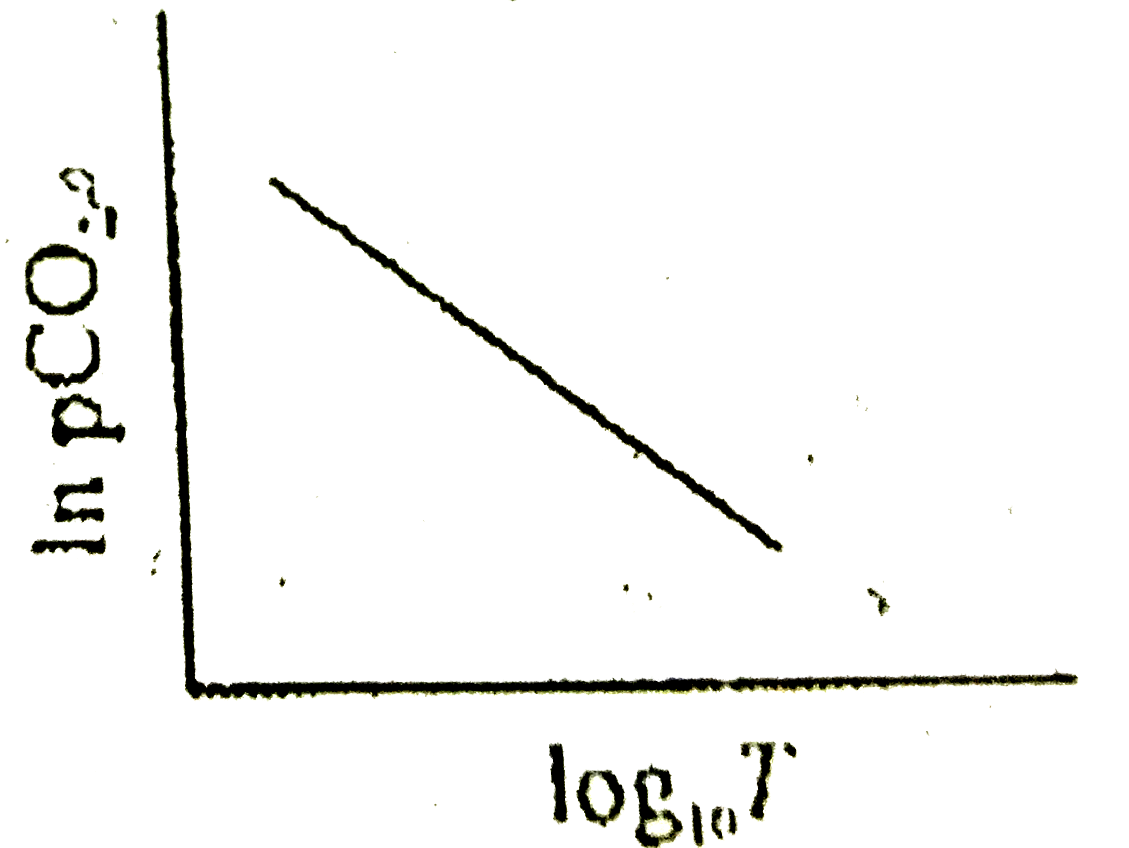
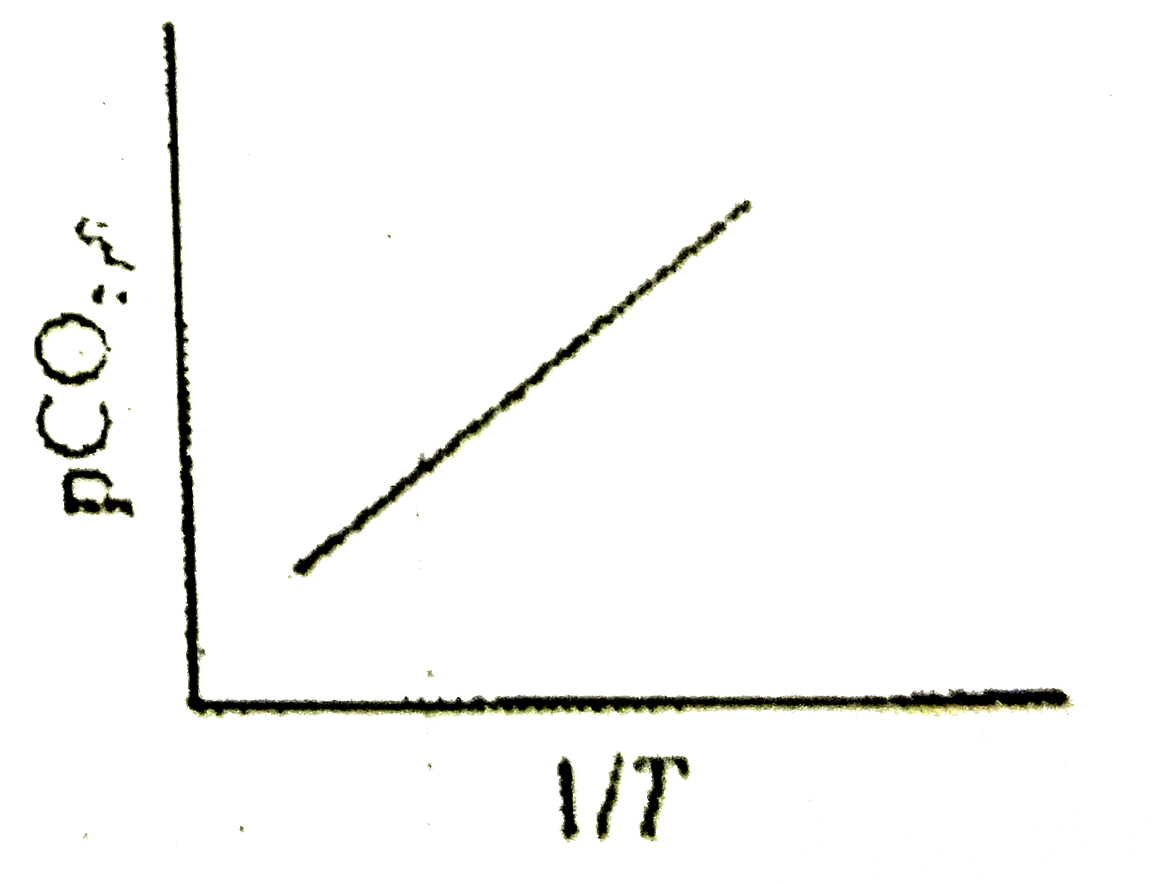
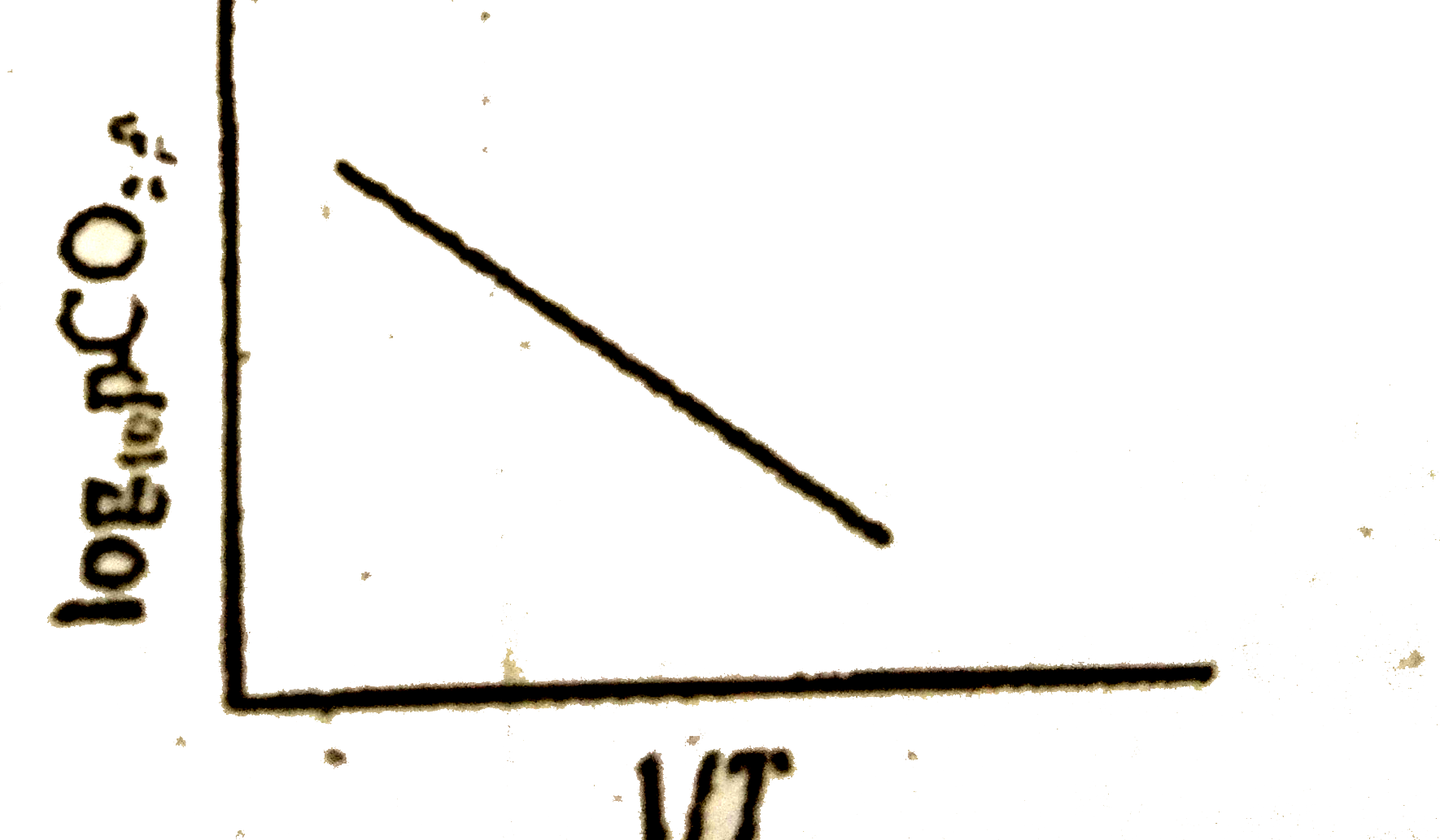A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the chemical equilibrium CaCO(3)(s)hArrCaO(s)+CO(2)(g),Delta(r)H^(...
Text Solution
|
- For the chemical equilibrium, CaCO(3)(s) hArr CaO(s)+CO(2)(g) Delta(r)...
Text Solution
|
- CaCO(3)(s) hArrCaO(s)+CO(2)(g) At equilibrium in the above case, 'a' m...
Text Solution
|
- For the reaction CaCO(3)(s)hArrCaO(s)+CO(2)(g) the equilibrium amount ...
Text Solution
|
- The thermal dissociation of equilibrium of CaCo(3)(s) is studied under...
Text Solution
|
- Write expression for K(p) "and"K(c) for the reaction CaCO(3)(S)harrCaO...
Text Solution
|
- CaCO(3)(s)hArrCaO(s)+CO(2)(g) CO(2)(g)hArrCO(g)+(1)/(2)O(2)(g) For abo...
Text Solution
|
- For the chemical equlibrium, CaCo(3(s))hArrCaO(s)+CO(2)(g),DeltaH(r)^(...
Text Solution
|
- CaCO(3)(s)hArrCaO(s)+CO(2)(g),DeltaH=110kJ The pressure of CO(2)
Text Solution
|