`CH_(3) CO_(2) C_(2) H_(5)` on reaction with sodium ethoxide in ethanol gives `A`, which on heating in the presence of acid gives `B` compound `B` is
`CH_(3) CO_(2) C_(2) H_(5)` on reaction with sodium ethoxide in ethanol gives `A`, which on heating in the presence of acid gives `B` compound `B` is
A
`CH_(3)COCH_(2)COOH`
B
`CH_(3)COCH_(3)`
C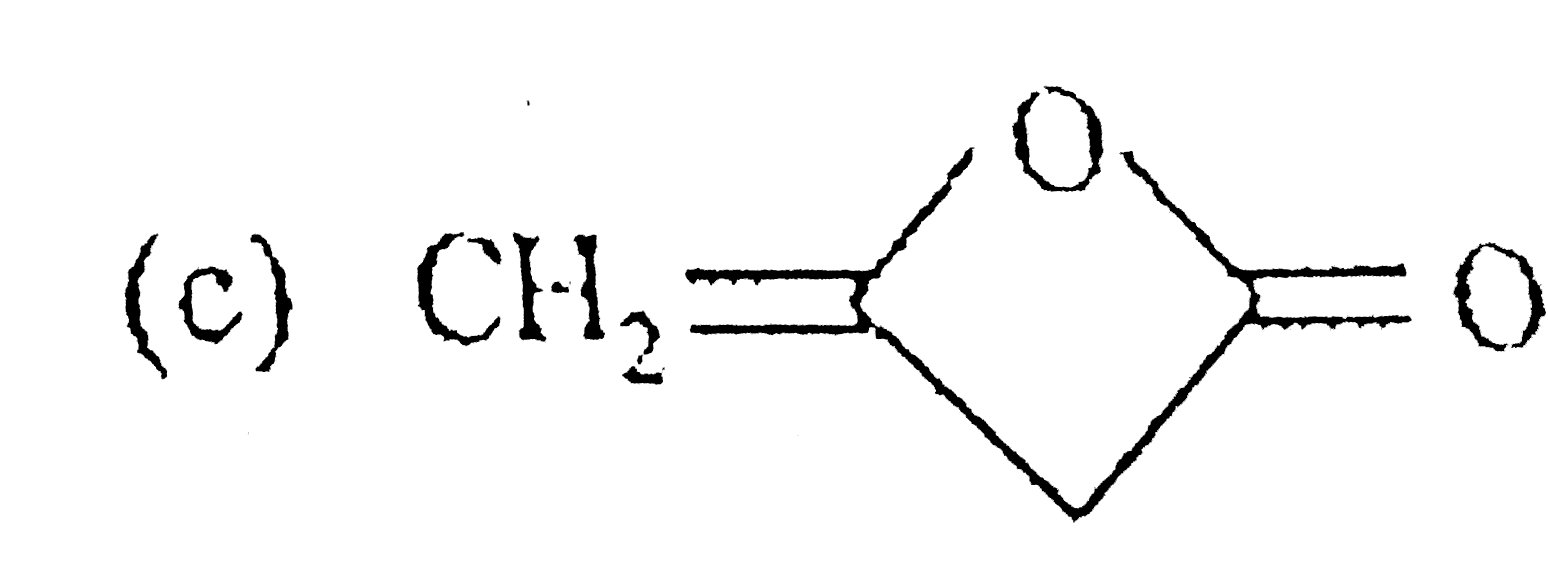
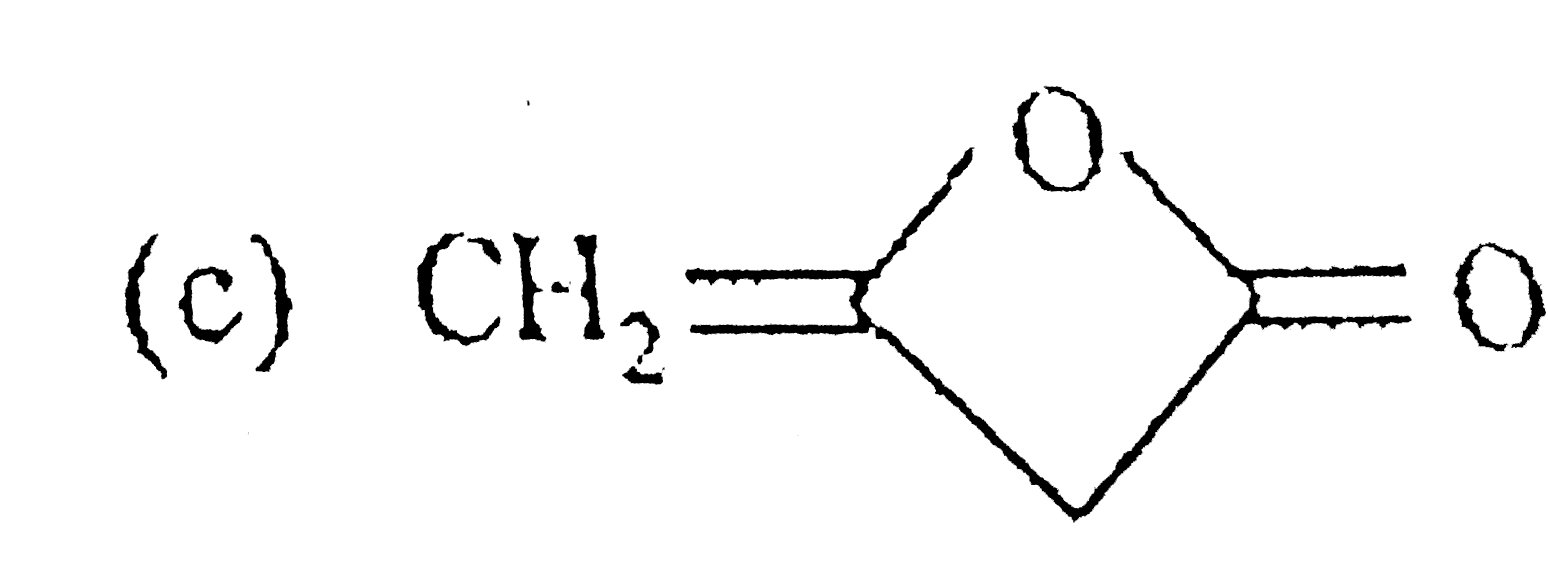
D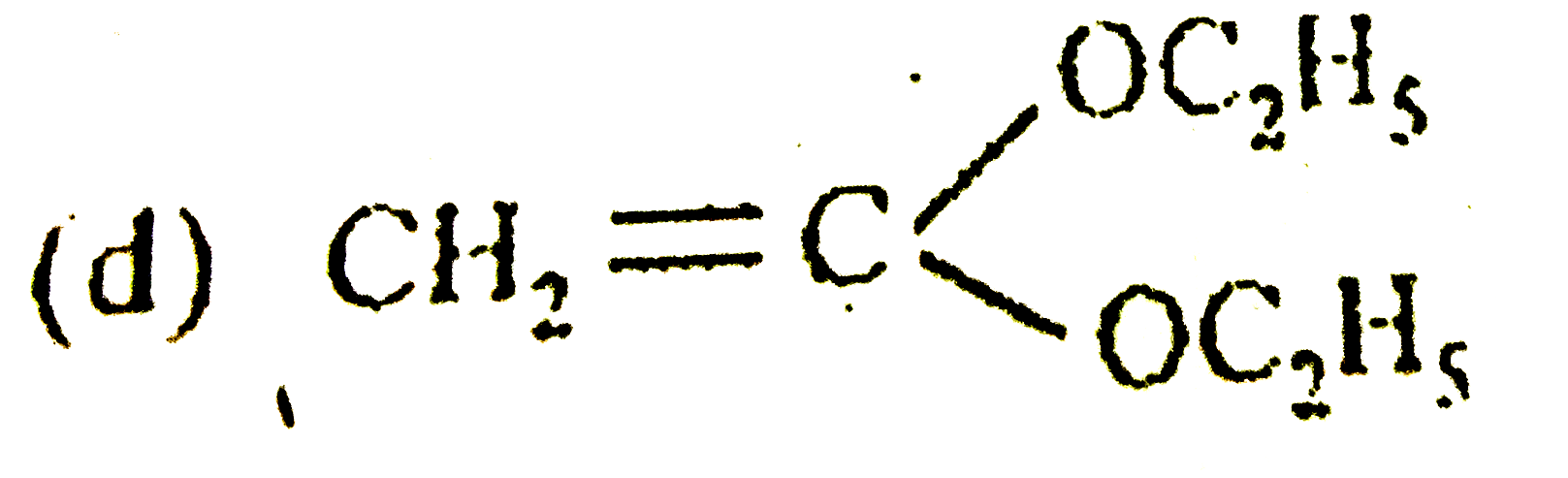
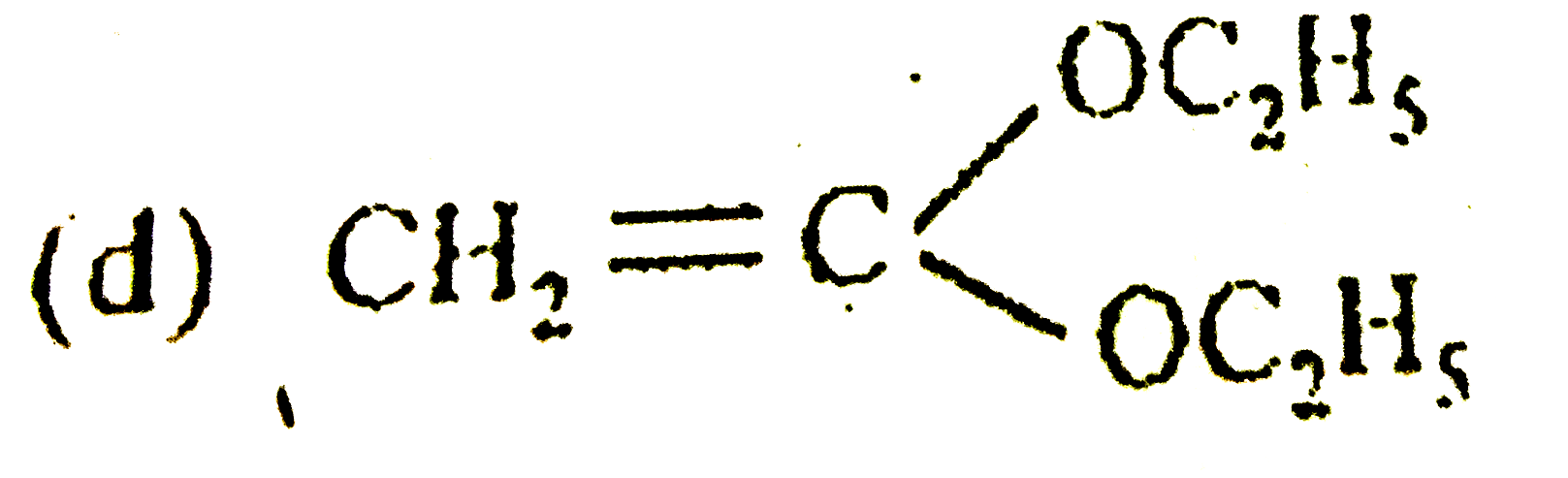
Text Solution
AI Generated Solution
The correct Answer is:
To solve the problem, we need to identify the compounds A and B formed in the reactions involving the ester CH₃COOC₂H₅ (ethyl acetate) with sodium ethoxide in ethanol, and then the subsequent reaction of compound A in the presence of acid.
**Step 1: Identify the starting compound.**
The starting compound is given as CH₃COOC₂H₅, which is ethyl acetate.
**Step 2: Reaction with sodium ethoxide.**
When ethyl acetate reacts with sodium ethoxide (C₂H₅ONa) in ethanol, it undergoes a condensation reaction. The sodium ethoxide acts as a base and deprotonates the hydrogen atom adjacent to the carbonyl group of the ester, leading to the formation of a β-keto ester.
The reaction can be represented as follows:
\[ \text{CH}_3COOC_2H_5 + \text{C}_2H_5ONa \rightarrow \text{CH}_3C(OH)(C_2H_5)COOC_2H_5 \]
This compound is labeled as A. The structure of compound A is:
\[ \text{CH}_3C(OH)(C_2H_5)COOC_2H_5 \]
**Step 3: Heating compound A in the presence of acid.**
When compound A is heated in the presence of acid, it undergoes dehydration and rearrangement to form a cyclic compound. The reaction leads to the formation of a compound that has a double bond and a cyclic structure.
The reaction can be represented as follows:
\[ \text{A} \xrightarrow{\text{heat, acid}} \text{B} \]
The structure of compound B can be represented as:
\[ \text{C}_5H_8O_2 \text{ (a cyclic compound)} \]
**Final Answer:**
- Compound A: CH₃C(OH)(C₂H₅)COOC₂H₅
- Compound B: A cyclic compound with a double bond, specifically a compound like 2,5-dimethyl-2,5-dihydrofuran.
To solve the problem, we need to identify the compounds A and B formed in the reactions involving the ester CH₃COOC₂H₅ (ethyl acetate) with sodium ethoxide in ethanol, and then the subsequent reaction of compound A in the presence of acid.
**Step 1: Identify the starting compound.**
The starting compound is given as CH₃COOC₂H₅, which is ethyl acetate.
**Step 2: Reaction with sodium ethoxide.**
When ethyl acetate reacts with sodium ethoxide (C₂H₅ONa) in ethanol, it undergoes a condensation reaction. The sodium ethoxide acts as a base and deprotonates the hydrogen atom adjacent to the carbonyl group of the ester, leading to the formation of a β-keto ester.
...
Similar Questions
Explore conceptually related problems
Compound (A) C_(5)H_(8)O_(2) liberated CO_(2) on reaction with sodium bicarbonate. It exists in two forms neither of which is optically active. It yielded compound (B) C_(5)H_(10)O_(2) on hydrogenation. Compound (B) can be separated into enantiomorphs. Write structures of (A) and (B).
Boron reacts with oxygen at 700^@C to give (A) . Compound (A) reacts with carbon and dry chloride to give (B) and carbon monoxide. (B) on reduction with LiAlH_(4) gives ( C) along with LiCl and AlCl_(3). ( C) on reaction with ammonia gives (D) . Which on heating gives (E).(C) on reaction with NaH gives (F) . Compound (A) is
An organic compound (A) (C_(6)H_(10)O) on reaction with CH_(3)MgBr followed by acid treatment gives compound (B). The compound (B) on ozonolysis gives compound (C ), which in the presence of a base gives 1-acetyl cyclopentene (D). The compound (B) on reaction with HBr gives compound (E ). Write the structures of (A), (B), (C ), (D), and (E ). Show how (D) is formed from (C ).
Assertion:2-Bromobutane on reaction with sodium ethoxide in ethanol gives 1-butene as a major product Reason:1-butene is more stable than 2-butene.
(i) An organic compound A with molecular formula C_(7)H_(8) on oxidation by chromyl chloride in the presence of C Cl_(4) gives a compound B which gives positive Tollen's test. The compound B on treatment with NaOH followed by acid hydrolysis gives two product C and D. C on oxidation gives B which on further oxidation gives D. the compound D on distillation with sodalime gives a hydrocarbon E. Below 60^(@)C , concentrated nitric acid reacts with E in the presence of concentrated sulphuric acid forming a compound F. identify the compounds A,B,C,D,E and F. (ii) Give chemical test to distinguish : Formaldehyde and acetaldehyde.
An aliphatic unsaturated hydrocarbon (A) when treated with HgSO//H_2SO_4 yields a compound (B) having molecular formula C_(3)H_(6)O . (B) on oxidation with concentrated HNO_3 gives two compounds (C) and (D). Compound (C) when treated with PCl_5 gives compound (E). (E) when reacts with ethanol gives a sweet-smelling liquid (F). Compound (F) is also formed when (C) reacts with ethanol in the presence of concentrated H_2SO_4 . (i) Identify the compound A, B, C, D, E and F. (ii) Give the chemical equation for the reaction of (C) with chlorine in the presence of red phosphorus and name the reaction.
A ketone A, which undergoes halform reaction, gives compound B on reduction B on heating with sulphuric acid gives compounds C, which forms mono-ozonide D.D on hydrolysis in the presence of zinc dust gives only acetaldehyde. Identify A, B and C. Write down the reaction involved.
An organic compound (A) C_(4)H_(9)Cl on reacting with aqueous KOH gives (B) and on reaction with alcoholic KOH gives (C ), which is also formed on passing the vapours of (B) over the heated copper. The compound (C ) readily decolourises bromine water. Ozonolysis of (C ) gives two compounds (D) and (E ). Compound (D) reacts with NH_(2)OH to give (F) and compound (E ) reacts with NaOH to have an alcohol (G) and sodium salt (H) of an acid. (D) can also be prepared form propyne on treatment with water in the presence of Hg^(2+) and H_(2)SO_(4) . Identify (A) to (H) with proper reasoning.
An acidic compound (A) (C_4 H_8 O_3) loses its optical activity on strong heating yielding (B) (C_4 H_6 O_2) which reacts readly with KMnO_4 . (B) forms a dervative ( C) with SOCl_2 which on reaction with (CH_3)_2 NH gives (D) . The compound (A) on oxidation with dilute chromic acid gives an unstable compound (E) which decarboxylates readily to give (F) (C_3 H_6 O) . The compound (F) gives a hydrocarbon (G) on treatment with amalgamated Zn and HCl . Give the structures of (A) to (G) with proper reasoning.
An organic compound A (C_(2)H_(6)O) reacts with sodium to form a compound B with the evolution of H_(2) and gives a yellow compound C on reacting with iodine and NaOH. When heated with conc. H_(2)SO_(4) at 413 K, it gives a compound D (C_(4)H_(10)O) which on reaction wiht conc. HI at 373 K gives compound E. The compound D is also obtained when B is heated with E. Idntify the compounds A,B,C,D and E write the equations for the reaction involved.
Recommended Questions
- CH(3) CO(2) C(2) H(5) on reaction with sodium ethoxide in ethanol give...
Text Solution
|
- CH(3) CO(2) C(2) H(5) on reaction with sodium ethoxide in ethanol give...
Text Solution
|
- CH(3)CO(2)C(2)H(5) on reaction with sodium ethoxide in ethanol gives X...
Text Solution
|
- Two mole of an ester (A) are condensed in presence of sodium ethoxide ...
Text Solution
|
- Cyclopentane on heating with bromine gives compound (A) which on react...
Text Solution
|
- Two moles of an ester (A) are condensed in the presence of sodium etho...
Text Solution
|
- The compounds 'A' undergoes Koble's reaction in the presence of CO(2) ...
Text Solution
|
- An aromatic compound A (C(2)H(6)O(2)) liberates hydrogen with metallic...
Text Solution
|
- An organic compound of molecula formula C(6)H(5)O"Na" is heated with C...
Text Solution
|