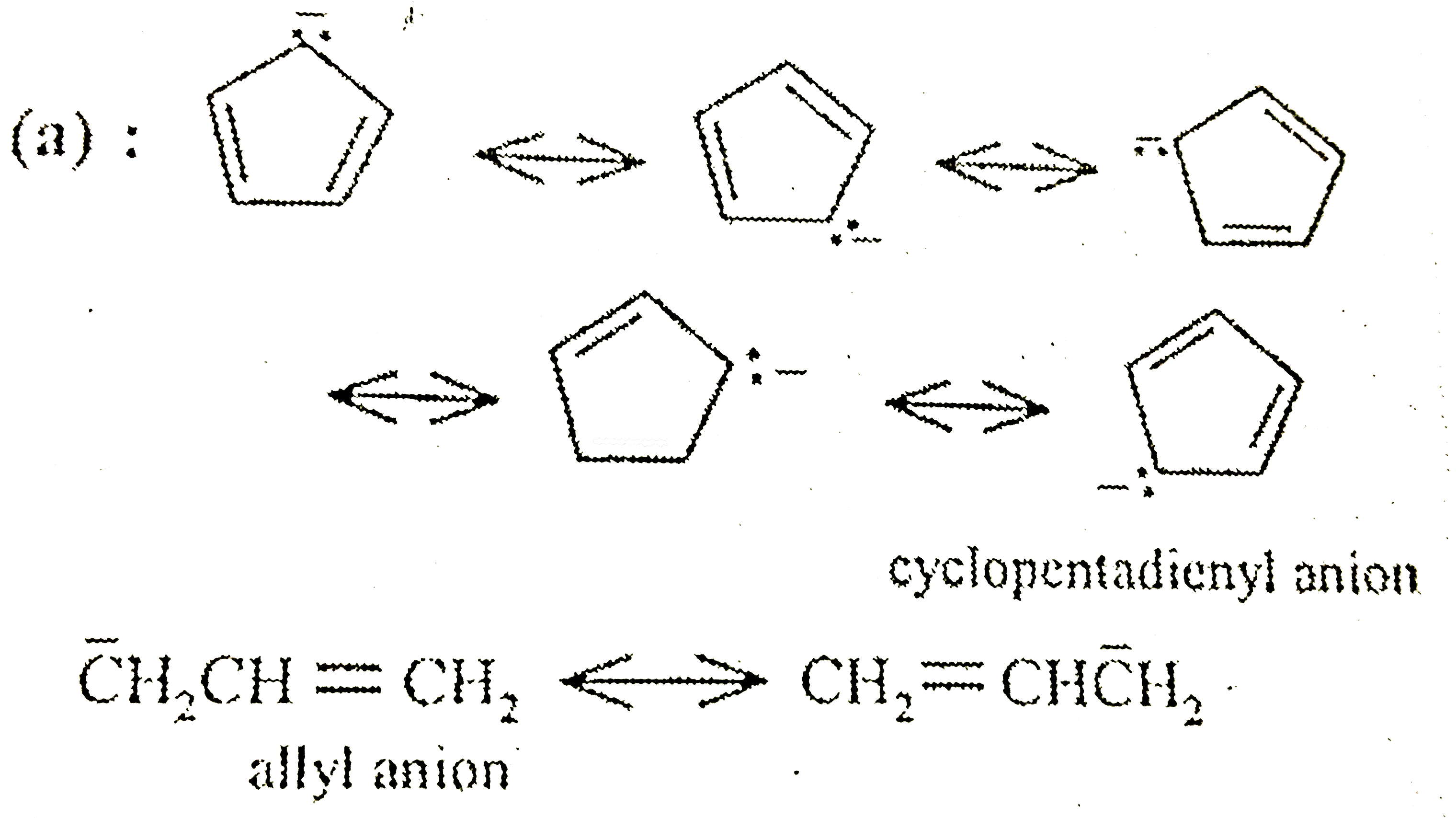A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Assertion: Cyclopentadienyl anion is aromatic in nature. Reason: Cyc...
Text Solution
|
- Describe the electron distribution in the Mos of the cyclopentadienyl ...
Text Solution
|
- (A) Cyclopentadienyl anion is aromatic. ( R) Aromatic molecules have...
Text Solution
|
- how many of the following species are aromatic in nature cyclopentadie...
Text Solution
|
- The cyclopentadienyl cation is while cyclopentadienyl anion is
Text Solution
|
- How many of the following species are aromatic in nature ? Cyclopentad...
Text Solution
|
- Assertion : Cyclopentadienyl anion is much more stable than allyl anio...
Text Solution
|
- Assertion : Cyclopentadienyl anion is much more stable than allyl anio...
Text Solution
|
- Assertion: Cyclopentadienyl anion is aromatic in nature. Reason: Cyclo...
Text Solution
|