A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
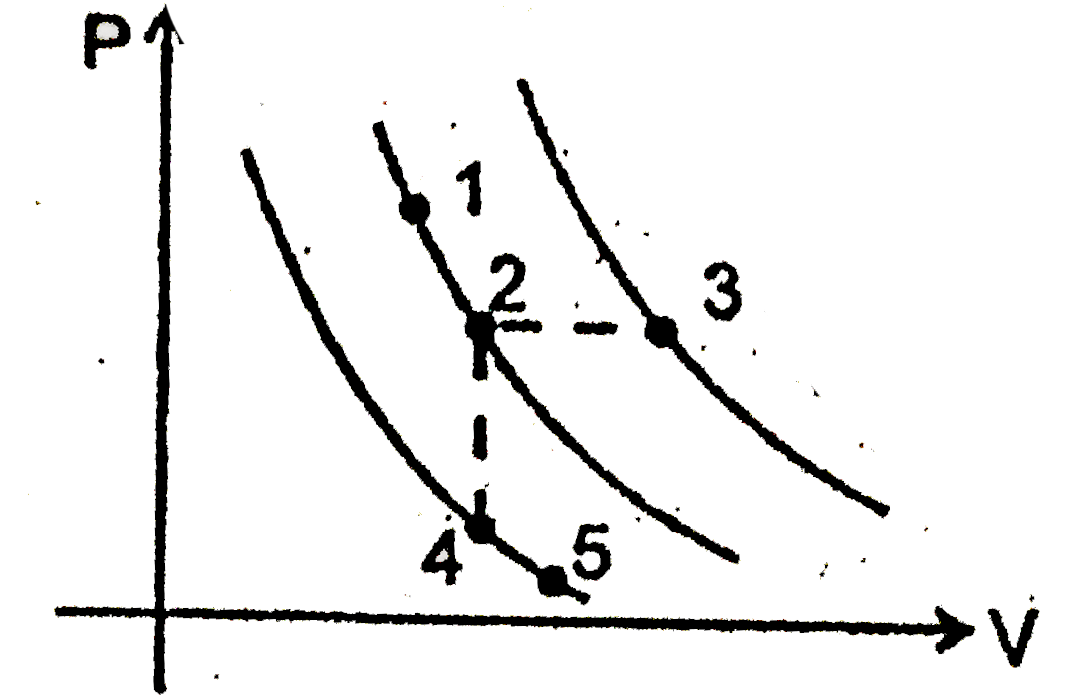
- A certain gas is taken to the five states representes by dots in the g...
Text Solution
|
- Five molecules of a gas have speed 2, 4, 6, 8 km//s . Calculate averag...
Text Solution
|
- The respective speeds of five molecules are 2,1.5,1.6,1.6 and 1.2 km/s...
Text Solution
|
- Suppose a container is evacuated to leave just one molecule of a gas i...
Text Solution
|
- A certain gas is taken to the five states representes by dots in the g...
Text Solution
|
- The following graphs are plotted for an ideal gas taken from state A t...
Text Solution
|
- The respective speeds of five molecules are 2, 1.5, 1.6, 1.6 and 1.2 k...
Text Solution
|
- পাঁচ রাজ্য শ্রেণিবিন্যাসের পাঁচ রাজ্য কী কী?
Text Solution
|
- माना कि bar(v), v(rms) तथा v(p) क्रमशः एकपरमाणुक आदर्श गैस में परमता...
Text Solution
|