Assertion : 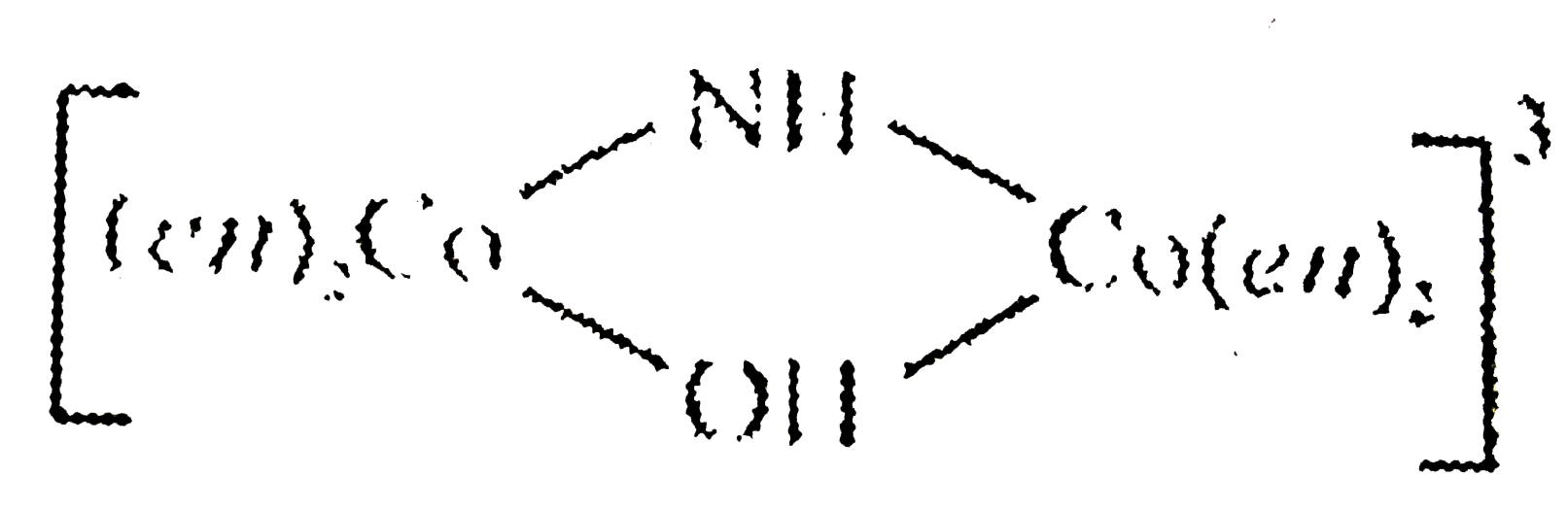 is named as tetrakis (ethylene- diammine) `mu-"hydroxo"-mu-"imido dicobalt (III) ion"`.
is named as tetrakis (ethylene- diammine) `mu-"hydroxo"-mu-"imido dicobalt (III) ion"`.
Reason : In naming polynuclear complexes i.e., containing two or more metal atoms joined by bridging ligands, the word `mu` is added with hyphen before the nane of such ligands.
Assertion :  is named as tetrakis (ethylene- diammine) `mu-"hydroxo"-mu-"imido dicobalt (III) ion"`.
is named as tetrakis (ethylene- diammine) `mu-"hydroxo"-mu-"imido dicobalt (III) ion"`.
Reason : In naming polynuclear complexes i.e., containing two or more metal atoms joined by bridging ligands, the word `mu` is added with hyphen before the nane of such ligands.
 is named as tetrakis (ethylene- diammine) `mu-"hydroxo"-mu-"imido dicobalt (III) ion"`.
is named as tetrakis (ethylene- diammine) `mu-"hydroxo"-mu-"imido dicobalt (III) ion"`. Reason : In naming polynuclear complexes i.e., containing two or more metal atoms joined by bridging ligands, the word `mu` is added with hyphen before the nane of such ligands.
A
a. If both assertion and reason are true and reason is the correct explanation of assertion
B
b. If both assertion and reason are true but reason is not the correct explanation of assertion
C
c.If assertion is true but reason is false
D
d. If both assertion and reason are false.
Text Solution
Verified by Experts
The correct Answer is:
A
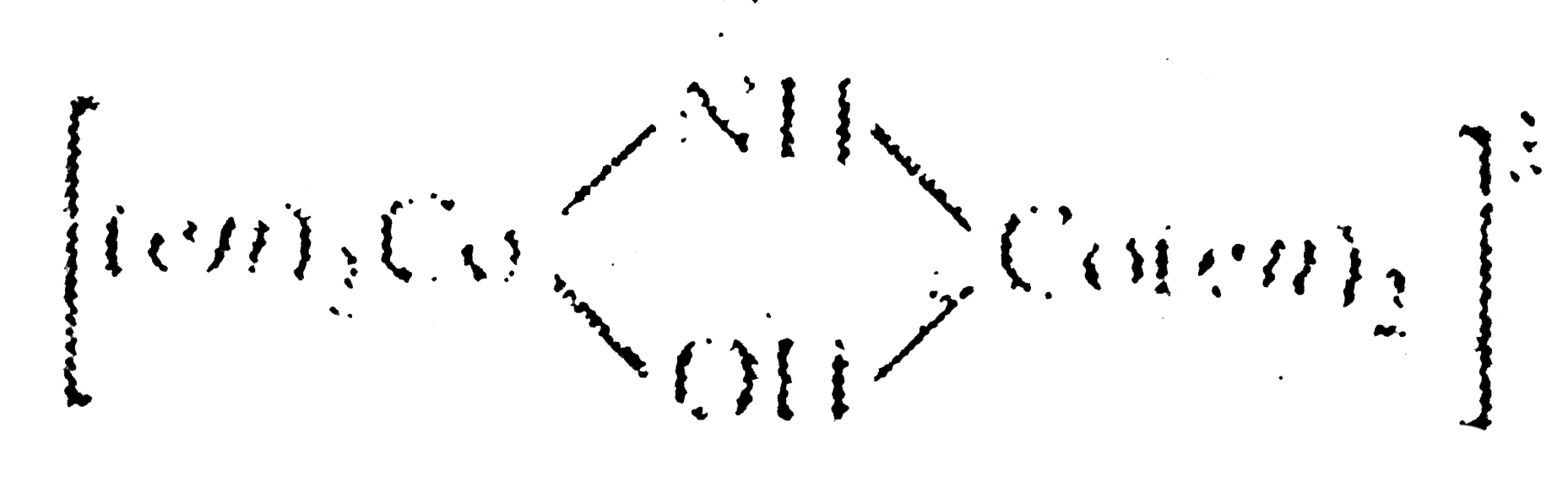 is named as tetrakis (ethylenediammine)-`mu`-hydroxo-`mu`-imido dicobalt (III) ion. For more than one bridging group the word u is repeated before cach bridging group.
is named as tetrakis (ethylenediammine)-`mu`-hydroxo-`mu`-imido dicobalt (III) ion. For more than one bridging group the word u is repeated before cach bridging group.Similar Questions
Explore conceptually related problems
The IUPAC name of [Co(H_2NCH_2CH_2NH_2)_2 (NO_2)NO_5 is bis(enthane-1,2-diamine) dinitrito-N-cobalt(III) nitrate.It follows the following rules that. (a)Cation is named first followed by anion. (b) Ligands are named in an alphabetical order before the name of the central atom/ion. ( c)Name of anionic ligands end in "o" (ex.chlorido) those of neutral (and if cation) ligands are the same except aqua for H_2O , ammine for NH_3 etc. (d)Prefixes mono,di,tri,etc.are used to indicate the number of individual ligand.When the name of the ligands include a numerical prefix, then terms bis, tris, tetrakis are used. (e)Oxidation state of the metal is indicated by Roman numeral in parenthesis. (f) For cationic and neutral complexes, the metal is named same as the element and for anionic complex, the name of metal ends with suffix-ate.For some metals, the Latin names are used in the complex anions , e.g., ferrate for Fe, argentate for silver etc. The correct IUPAC name of the coordination compound [Pt(NH_3)_2Cl(NH_2CH_3)] is :
The IUPAC name of [Co(H_2NCH_2CH_2NH_2)_2 (NO_2)NO_5 is bis(enthane-1,2-diamine) dinitrito-N-cobalt(III) nitrate.It follows the following rules that. (a)Cation is named first followed by anion. (b) Ligands are named in an alphabetical order before the name of the central atom/ion. ( c)Name of anionic ligands end in "o" (ex.chlorido) those of neutral (and if cation) ligands are the same except aqua for H_2O , ammine for NH_3 etc. (d)Prefixes mono,di,tri,etc.are used to indicate the number of individual ligand.When the name of the ligands include a numerical prefix, then terms bis, tris, tetrakis are used. (e)Oxidation state of the metal is indicated by Roman numeral in parenthesis. (f) For cationic and neutral complexes, the metal is named same as the element and for anionic complex, the name of metal ends with suffix-ate.For some metals, the Latin names are used in the complex anions , e.g., ferrate for Fe, argentate for silver etc. The IUPAC name of Fe_4[Fe(CN)_6]_3 is :
The IUPAC definition of a transition element is that it is an element that has an incomplete d-subshell in either the neutral atom or its ion. Thus the group 12 elements are member of the d-block but are not transition elements. Chemically solft members of the d-block occurs as sulphide minerals and are partially oxidised to obtain the metal, the more electropositive 'hard' metals occurs as oxides and are extracted by reduction. Opposite to p-block elements, the higher oxidation states are favoured by the heavier elements of d-block Metals on the right of the d-block tend to exist in low oxidation states and form complexes with the ligands. Square-planar complexes are common for the platinum metals and gold in oxidation states that yield d^8 electronic configuration, which include RH(I),Ir(I),Pd(II),Pt(II) and Au(III). The most distinctive features/properties of transition metal complex is their wide range of colours.The crystal field theory attributes the colour of the coordination compounds to d-d transition of the electron.It is important to note that (a) in absence of ligand, crystal field spilling does not occur and hence the substances is colourless, (b) the type of ligand also influences the colour of the complexes. Which of the following has dsp^2 hybridisation and is diamagnetic in nature ? (i) Na_4[Cr(CO)_4] , (ii) [Ni(DMGH)_2] , (iii) [PtHBr(PEt_3)_2] (iv) [As(SCN)_4]^(3-) , (v) [AuBr_4]^(-)
coordination compounds often show various types of isomerism. The isomerism can be categorized in two main types (a) structural isomerism (b) stereo or space isomerism Structural isomerism arises due to the difference in structures of coordination-sempounds while stereo or space isomerism arises on account of the different positions and arrangements of ligands (atoms or groups) in space around the metal lonStructural isomerism can be classified in following types (i) tonization isomers- which give different ions in solution, e.g. [CoBr(NH_(3))_(5)]]SO_(4) and [Co(SO_(4))_(5)(NH_(3))_(5)]Br Hydrate isomers which differ in H_(2)O as ligand or as hydration, e.g. [Cr(H_(2)O)_(5)]CI_(2)(H_(2)O)]Cl_(2).H_(2)O[CrCl_(2)(H_(2)O)]CI.2H_(2)O (iii) Linkage isomers, which differ in atom linked to 'metal atom, e.g. [CO(NO_(2))(NH_(3))_(5)]^(2+) and CO(ONO)(NH_(3))_(6)]^(2+) Coordination isomers- which involve interchange of ligands, e.g. [Co(NH_(3))_(6)] [Cr(CN)_(6)] and [Cr(NH_(3))_(6)] [Co(CN)_(6)] (v) Coordination position isomerism-which arises in the bridged complexes due to the difference in the attachment of ligands with the metal atoms QThe total number of possible isomers for the complex compound [Cu^(ll)(NH_(3))_(4)] [Pt^(ll)Cl_(4) ]are
coordination compounds often show various types of isomerism. The isomerism can be categorized in two main types (a) structural isomerism (b) stereo or space isomerism Structural isomerism arises due to the difference in structures of coordination-sempounds while stereo or space isomerism arises on account of the different positions and arrangements of ligands (atoms or groups) in space around the metal lonStructural isomerism can be classified in following types (i) tonization isomers- which give different ions in solution, e.g. [CoBr(NH_(3))_(5)]]SO_(4) and [Co(SO_(4))_(5)(NH_(3))_(5)]Br Hydrate isomers which differ in H_(2)O as ligand or as hydration, e.g. [Cr(H_(2)O)_(5)]CI_(2)(H_(2)O)]Cl_(2).H_(2)O[CrCl_(2)(H_(2)O)]CI.2H_(2)O (iii) Linkage isomers, which differ in atom linked to 'metal atom, e.g. [CO(NO_(2))(NH_(3))_(5)]^(2+) and CO(ONO)(NH_(3))_(6)]^(2+) Coordination isomers- which involve interchange of ligands, e.g. [Co(NH_(3))_(6)] [Cr(CN)_(6)] and [Cr(NH_(3))_(6)] [Co(CN)_(6)] (v) Coordination position isomerism-which arises in the bridged complexes due to the difference in the attachment of ligands with the metal atoms Q Which of the following coordination compounds, exhibits ionization isomerism
coordination compounds often show various types of isomerism. The isomerism can be categorized in two main types (a) structural isomerism (b) stereo or space isomerism Structural isomerism arises due to the difference in structures of coordination-sempounds while stereo or space isomerism arises on account of the different positions and arrangements of ligands (atoms or groups) in space around the metal lonStructural isomerism can be classified in following types (i) tonization isomers- which give different ions in solution, e.g. [CoBr(NH_(3))_(5)]]SO_(4) and [Co(SO_(4))_(5)(NH_(3))_(5)]Br Hydrate isomers which differ in H_(2)O as ligand or as hydration, e.g. [Cr(H_(2)O)_(5)]CI_(2)(H_(2)O)]Cl_(2).H_(2)O[CrCl_(2)(H_(2)O)]CI.2H_(2)O (iii) Linkage isomers, which differ in atom linked to 'metal atom, e.g. [CO(NO_(2))(NH_(3))_(5)]^(2+) and CO(ONO)(NH_(3))_(6)]^(2+) Coordination isomers- which involve interchange of ligands, e.g. [Co(NH_(3))_(6)] [Cr(CN)_(6)] and [Cr(NH_(3))_(6)] [Co(CN)_(6)] (v) Coordination position isomerism-which arises in the bridged complexes due to the difference in the attachment of ligands with the metal atoms QThe pair [CO(NH_(3))_(5)NO_(3)]SO_(4) and [CO(NH_(3))SO_(4)]NO_(3) will exhibit
coordination compounds often show various types of isomerism. The isomerism can be categorized in two main types (a) structural isomerism (b) stereo or space isomerism Structural isomerism arises due to the difference in structures of coordination-sempounds while stereo or space isomerism arises on account of the different positions and arrangements of ligands (atoms or groups) in space around the metal lonStructural isomerism can be classified in following types (i) tonization isomers- which give different ions in solution, e.g. [CoBr(NH_(3))_(5)]]SO_(4) and [Co(SO_(4))_(5)(NH_(3))_(5)]Br Hydrate isomers which differ in H_(2)O as ligand or as hydration, e.g. [Cr(H_(2)O)_(5)]CI_(2)(H_(2)O)]Cl_(2).H_(2)O[CrCl_(2)(H_(2)O)]CI.2H_(2)O (iii) Linkage isomers, which differ in atom linked to 'metal atom, e.g. [CO(NO_(2))(NH_(3))_(5)]^(2+) and CO(ONO)(NH_(3))_(6)]^(2+) Coordination isomers- which involve interchange of ligands, e.g. [Co(NH_(3))_(6)] [Cr(CN)_(6)] and [Cr(NH_(3))_(6)] [Co(CN)_(6)] (v) Coordination position isomerism-which arises in the bridged complexes due to the difference in the attachment of ligands with the metal atoms QThe compounds [Cr(H_(2)O_(6)]Cl_(3)[Cr(H_(2)O)_(5)CI]Cl_(2).H_(2)O and [Cr(H_(2)O)_(4)Cl_(2)]Cl2H_(2)O exhibit
coordination compounds often show various types of isomerism. The isomerism can be categorized in two main types (a) structural isomerism (b) stereo or space isomerism Structural isomerism arises due to the difference in structures of coordination-sempounds while stereo or space isomerism arises on account of the different positions and arrangements of ligands (atoms or groups) in space around the metal lonStructural isomerism can be classified in following types (i) tonization isomers- which give different ions in solution, e.g. [CoBr(NH_(3))_(5)]]SO_(4) and [Co(SO_(4))_(5)(NH_(3))_(5)]Br Hydrate isomers which differ in H_(2)O as ligand or as hydration, e.g. [Cr(H_(2)O)_(5)]CI_(2)(H_(2)O)]Cl_(2).H_(2)O[CrCl_(2)(H_(2)O)]CI.2H_(2)O (iii) Linkage isomers, which differ in atom linked to 'metal atom, e.g. [CO(NO_(2))(NH_(3))_(5)]^(2+) and CO(ONO)(NH_(3))_(6)]^(2+) Coordination isomers- which involve interchange of ligands, e.g. [Co(NH_(3))_(6)] [Cr(CN)_(6)] and [Cr(NH_(3))_(6)] [Co(CN)_(6)] (v) Coordination position isomerism-which arises in the bridged complexes due to the difference in the attachment of ligands with the metal atoms Qcoordinate isomerism 33. How many hydrate isomers are possible with the formulae CrCl_(3).6H_(2)O
When degenerate d-orbitals of an isolated atom/ion come under influence of magnetic field of ligands, the degeneray is lost. The two set t_(2g)(d_(xy),d_(yz),d_(xz)) and e_(g) (d_(x^(2))-d_(x^(2)-y^(2)) are either stabilized or destabilized depending upon the nature of magnetic field. it can be expressed diagrammatically as: Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for tetrahedral complexes, Delta is about 4/9 times to Delta_(0) (CFSE for octahedral complex). this energy lies in visible region and i.e., why electronic transition are responsible for colour. such transition are not possible with d^(0) and d^(10) configuration. Q. Which of the following is correct arrangement of ligand in terms of the Dq values of their complexes with any particularr 'hard' metal ion:
When degenerate d-orbitals of an isolated atom/ion come under influence of magnetic field of ligands, the degeneray is lost. The two set t_(2g)(d_(xy),d_(yz),d_(xz)) and e_(g) (d_(x^(2))-d_(x^(2)-y^(2)) are either stabilized or destabilized depending upon the nature of magnetic field. it can be expressed diagrammatically as: Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for tetrahedral complexes, Delta is about 4/9 times to Delta_(0) (CFSE for octahedral complex). this energy lies in visible region and i.e., why electronic transition are responsible for colour. such transition are not possible with d^(0) and d^(10) configuration. Q. Which of the following is correct arrangement of ligand in terms of the Dq values of their complexes with any particularr 'hard' metal ion:
Recommended Questions
- Assertion : is named as tetrakis (ethylene- diammine) mu-"hydroxo"-mu...
Text Solution
|
- In octaamine -mu -dihydroxodiiron(III)sulphate the number of bridging ...
Text Solution
|
- Assertion (A) Toxic metal ions are removed by the chelating ligands. R...
Text Solution
|
- Wht will be the theoretical value of magnetic moment (mu) when CN^(-) ...
Text Solution
|
- Assertion : is named as tetrakis ( ethylene diamine ) mu- hydroxo - i...
Text Solution
|
- Assertion : is named as tetrakis (ethylene- diammine) mu-"hydroxo"-mu-...
Text Solution
|
- Assertion : Toxic metal ions are removed by the chelating ligands Reas...
Text Solution
|
- Name the type of isomerism when ambidentate ligands are attached to ce...
Text Solution
|
- Assertion (A) : Toxic metal ions are removed by chelating ligands. Rea...
Text Solution
|