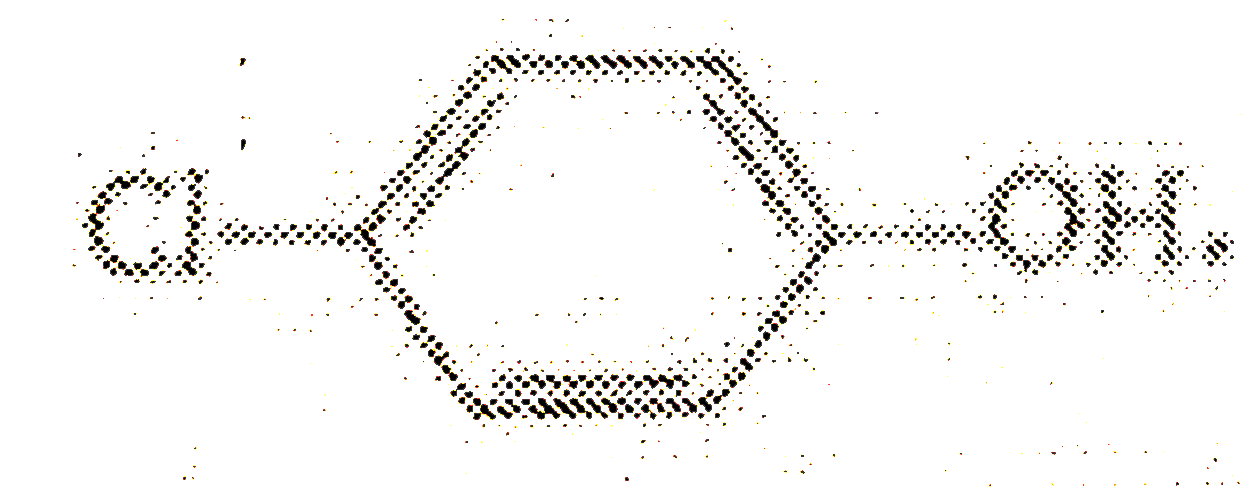
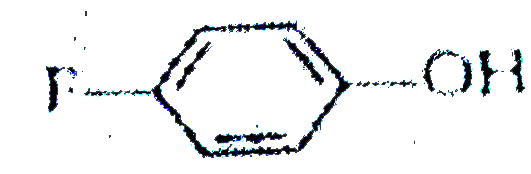
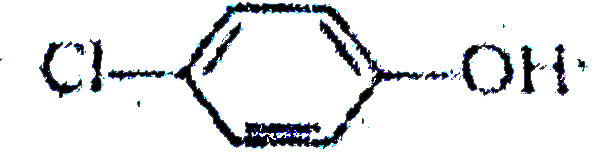
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Assertion: is less acidic than Reason: -F exerts better+mesomeri...
Text Solution
|
- Assertion: F is more electronegative than Cl . Reason: F has high elec...
Text Solution
|
- Assertion :- I effect of Cl atom is greater than F atom. Reason :- C...
Text Solution
|
- Assertion :- The following reaction C-Cl+MFrarrC-F+M-Cl Proceed better...
Text Solution
|
- Assertion: EA1 of fluorine is less than that of chlorine. Reason: Ad...
Text Solution
|
- Assertion: F-F bond in F2 is stronger than Cl-Cl bond in Cl2 Reason:...
Text Solution
|
- Assertion: F atom has lower electron affinity than Cl atom. Reason: Ad...
Text Solution
|
- Assertion: is less acidic than Reason: -F exerts better+mesomeric effe...
Text Solution
|
- Assertion: F atom has less electron afffinity than Cl atom. Reason: Ad...
Text Solution
|
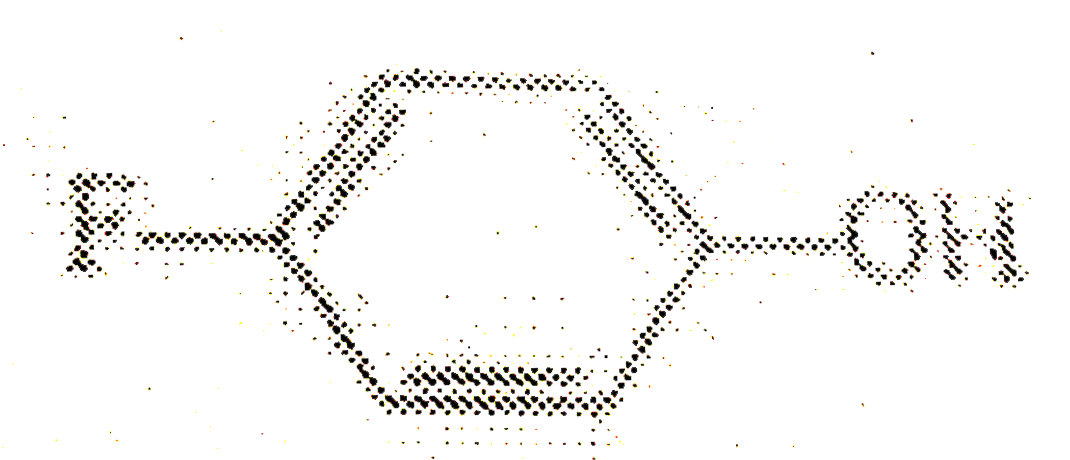 is less acidic than
is less acidic than