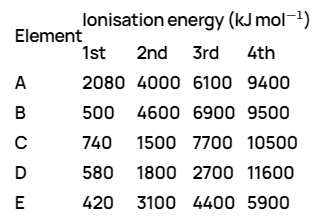
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Following table shows the successive molar ionization energy (" kJ mol...
Text Solution
|
- The first five ionization energies of an element are 801,2428,3660,250...
Text Solution
|
- Ionization energies of five elements of same period in kcal/mol are gi...
Text Solution
|
- Ionization energies of five elements of same period in kcal/mol are gi...
Text Solution
|
- Which is most similar for the elements in a group in the periodic tabl...
Text Solution
|
- An element has successive ionization enthalpies as 940 (first),2080,30...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: ...
Text Solution
|
- The table gives the first four ionization energies in kJ mol^(-1) of f...
Text Solution
|
- An element has successive ionization enthalpies as 940 (first),2080,30...
Text Solution
|