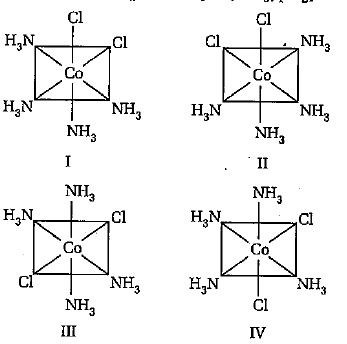
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the following arrnagements of the octahedral complex ion [Co(...
Text Solution
|
- Arrange the following complexes in the increasing order of conductivit...
Text Solution
|
- The number of ions given by the complex compound [Co(NH(3))(4)Cl(2)]Cl...
Text Solution
|
- The ion [Co(NH(3))(6)]^(+) is octahedral and high spin. This complex i...
Text Solution
|
- Arrange the following complexes in order of increasing electrical cond...
Text Solution
|
- Arrange the following complexes in the increasing order of conductivit...
Text Solution
|
- How many different isomers exist for the octahedral complex [Co(NH(3))...
Text Solution
|
- How many different isomers exist for the octahedral complex [Co(NH(3))...
Text Solution
|
- [CO(NH(3))(4)Cl(2)]Cl संकुल द्वारा जल में दिए गए कुल आयनों की संख्या.....
Text Solution
|