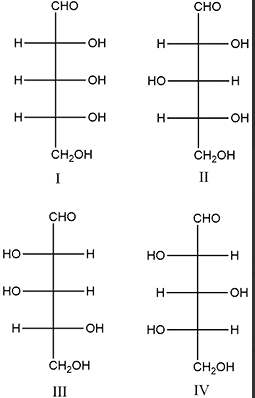
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Two aldopentoses A and B given the same osazone derivative A is oxidiz...
Text Solution
|
- Which of the following alpha -amino acids is not optically active?
Text Solution
|
- Two aldopentoses X and Y give the same osazone derivative. X is oxidiz...
Text Solution
|
- Which of the following gives an optically inactive aldaric acid on oxi...
Text Solution
|
- Which of the following amino acids is not optically active
Text Solution
|
- Which of the following acid is optically active ?
Text Solution
|
- Which amino acid is not optically active?
Text Solution
|
- Which configuration (R or S) does the bottom asymmetric carbon have fo...
Text Solution
|
- The amino acid which is not optically active is
Text Solution
|