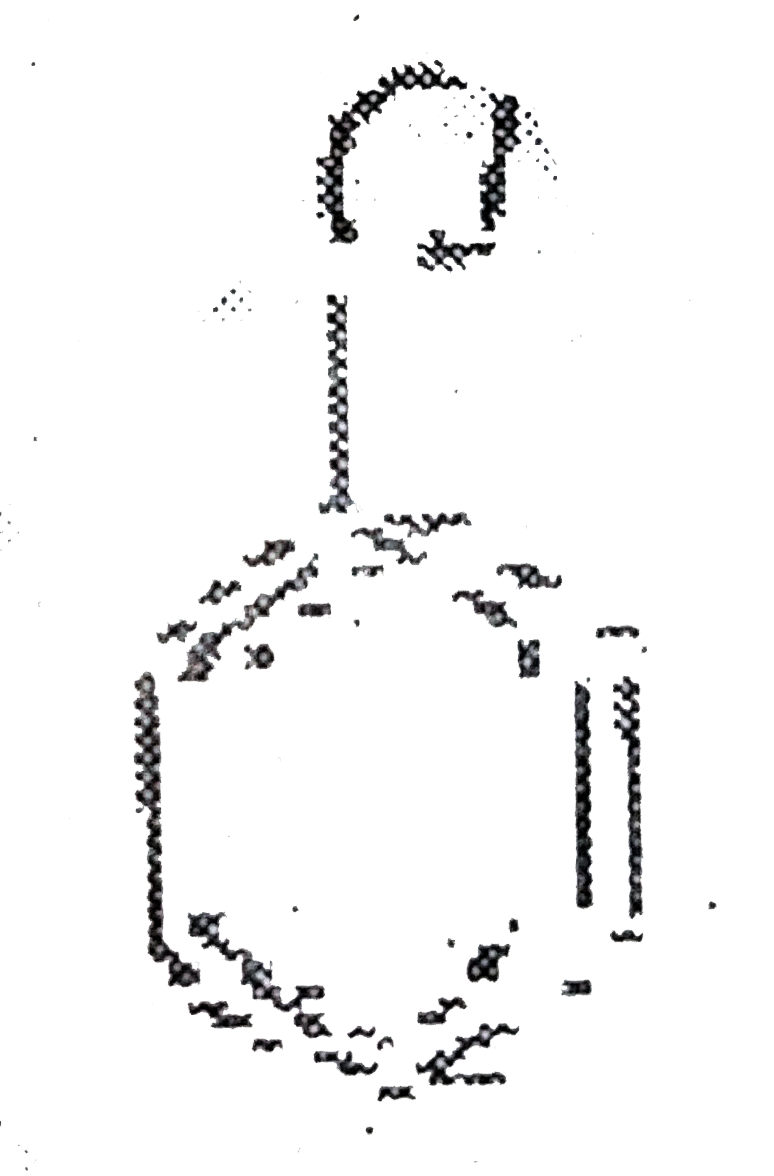
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which one of the following does not give white precipitate with acidif...
Text Solution
|
- Which of the following will not give white precipitate with ammoniacal...
Text Solution
|
- Which one of the following will not goves white precipitate with ammon...
Text Solution
|
- Which of the following will not give white precipitate with ammoniacal...
Text Solution
|
- Which one of the following will not give white precipitate with ammoni...
Text Solution
|
- Which of the following compound gives white precipitate with silver ni...
Text Solution
|
- Which of the following compound(s) give white precipitate with silver ...
Text Solution
|
- Which compound does not give precipitate with ammonical silver nitrate...
Text Solution
|
- Which one of the following does not give white precipitate with acidif...
Text Solution
|