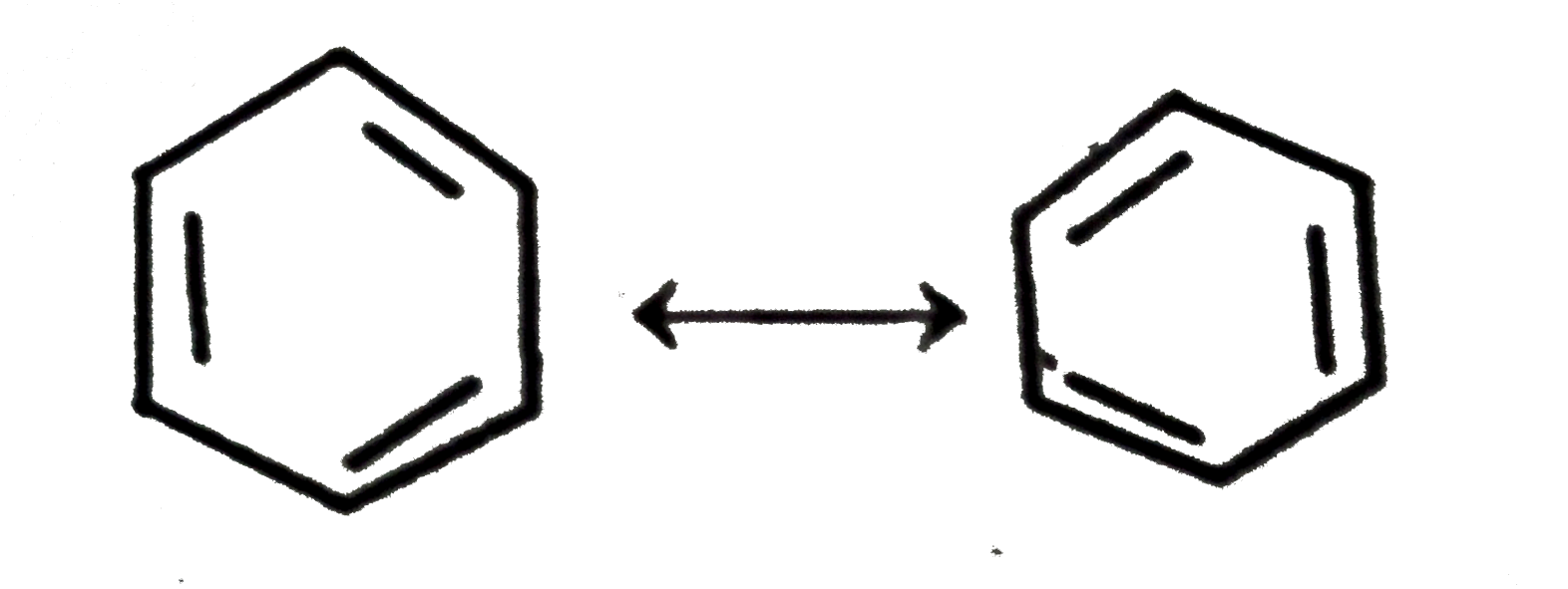
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Assertion : Benzene exhibit tow different bond length , due to C...
Text Solution
|
- C-C bond length in benzene is
Text Solution
|
- C-C bond length in benzene is
Text Solution
|
- C-C' bond length in benzene lies between single and double bond. The r...
Text Solution
|
- Benzene ring contains alternate single and double bonds, yet alll the ...
Text Solution
|
- The length of C-C bonds in benzene is
Text Solution
|
- Assertion : Benzene exhibit tow different bond length , due to C...
Text Solution
|
- C-C' bond length in benzene lies between single and double bond. The r...
Text Solution
|
- C-C bond length in benzene is
Text Solution
|