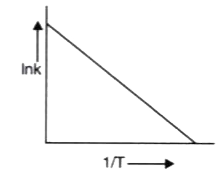
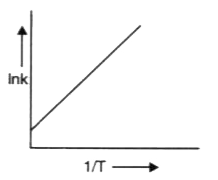
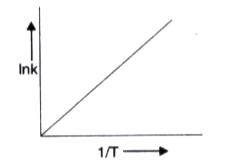
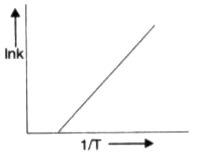
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
ICSE|Exercise EXERCISE (PART- I (OBJECTIVE QUESRTIONS) C. CORRECT THE FOLLOWING STATEMENTS BY CHANGING THE UNDERLINED PART OF THE STATEMENT ( DO NOT CHANGE THE WHOLE SENTENCE .)) |39 VideosCHEMICAL KINETICS
ICSE|Exercise EXERCISE (PART- I (OBJECTIVE QUESRTIONS) D. MATCH THE FOLLOWING ) |3 VideosCHEMICAL KINETICS
ICSE|Exercise EXERCISE (PART- I (OBJECTIVE QUESRTIONS)A.FILL IN THE BLANKS) |54 VideosBIOMOLECULES
ICSE|Exercise ISC EXAMINATION QUSTIONS (PART-I)(DESCRIPTIVE QUESTIONS)|21 VideosCHEMISTRY IN EVERYDAY LIFE
ICSE|Exercise EXERCISE (PART - II (DESCRIPTIVE QUESTIONS) (LONG ANSWER QUESTIONS))|19 Videos
Similar Questions
Explore conceptually related problems
ICSE-CHEMICAL KINETICS-EXERCISE (PART- I (OBJECTIVE QUESRTIONS)B.COMPLE THE FOLLOWING STATEMENTS BY SELECTING THE CORRECT ALTERNATIVE FROM THE CHOICES GIVEN )
- Consider the given figure and mark the correct option.
Text Solution
|
- Consider a first order gas phase decomposition reaction given below: ...
Text Solution
|
- According to Arrhenius equation, rate constant k is equal to A e^(- Ea...
Text Solution
|
- Consider the Arrhenius equation given below and mark the correct op...
Text Solution
|
- A graph of volume of hydrogen released vs time for the reaction betwee...
Text Solution
|
- Which of the following statements is not correct about order of a reac...
Text Solution
|
- Consider the graph given in figure . Which of the following optio...
Text Solution
|
- Which of the following statement is correct?
Text Solution
|
- Which of the following expressions is correct for the rate of reaction...
Text Solution
|
- Which of the following graphs represents exothermic reaction ?
Text Solution
|
- Rate law for the reaction A + 2B to C is found to be Rate =k[A][B] Con...
Text Solution
|
- Which of the following statements is incorrect about the collison theo...
Text Solution
|
- A first order reaction is 50% completed in 1.26 xx 10^(14)s. How much ...
Text Solution
|
- Compounds 'A' and 'B' react according to the following chemical eq...
Text Solution
|
- Which of the following statements is not correct for the catalyst ?
Text Solution
|
- The value of rate constant of a pseudo first order reaction
Text Solution
|
- Consider the reaction A to B. The concentration of both the reactan...
Text Solution
|
- The time of completion of 90% of a first order reaction is approximate...
Text Solution
|
- The rate law for the reaction 2N2 O5 to 4NO2 + O2 is
Text Solution
|
- At any stage of the reaction 3A to2B, the reaction rate +(dB )/(dt) wi...
Text Solution
|