Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL KINETICS
ICSE|Exercise EXERCISE (PART- II (DESCRIPTIVE QUESRTIONS) B. SHORT ANSWER QUESTIONS ) |119 VideosCHEMICAL KINETICS
ICSE|Exercise EXERCISE (PART- II (DESCRIPTIVE QUESRTIONS) C.LONG ANSWER QUESTIONS ) |54 VideosCHEMICAL KINETICS
ICSE|Exercise EXERCISE (PART- I (OBJECTIVE QUESRTIONS) D. MATCH THE FOLLOWING ) |3 VideosBIOMOLECULES
ICSE|Exercise ISC EXAMINATION QUSTIONS (PART-I)(DESCRIPTIVE QUESTIONS)|21 VideosCHEMISTRY IN EVERYDAY LIFE
ICSE|Exercise EXERCISE (PART - II (DESCRIPTIVE QUESTIONS) (LONG ANSWER QUESTIONS))|19 Videos
Similar Questions
Explore conceptually related problems
ICSE-CHEMICAL KINETICS-EXERCISE (PART- II (DESCRIPTIVE QUESRTIONS) A. VERY SHORT ANSWER QUESTIONS (WITH ANSWERS ))
- How is half-life period of a reaction is inversely proportional to ini...
Text Solution
|
- In some cases, it is found that large number of colliding molecules ha...
Text Solution
|
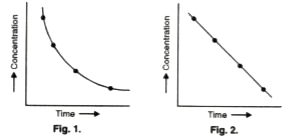
- In the reaction Ato B , if the concentration of A is plotted against t...
Text Solution
|
- What is the rate determining step of a reaction?
Text Solution
|
- The kinetics for the reaction, 2NO+2H2 to N2 + 2H2O is explained by t...
Text Solution
|
- Write the rate law and order for the following reaction: AB2 +C2 ...
Text Solution
|
- Define Elementary step in a reaction
Text Solution
|
- What is the temperature coefficient?
Text Solution
|
- What are effective collisions ?
Text Solution
|
- Define threshold energy.
Text Solution
|
- What is the effect of catalyst on the rate of reaction ?
Text Solution
|
- Define activation energy of a reaction.
Text Solution
|
- How is activation energy of a reaction affected? by using a catalys...
Text Solution
|
- How is activation energy of a reaction affected by increasing the te...
Text Solution
|
- In some cases, it is found that large number of colliding molecules ha...
Text Solution
|
- The reaction 2 H2(g) + O2(aq) to 2 H2 O(l) is thermodynamically feasi...
Text Solution
|
- The activation energy of a reaction is zero. Will the rate constant of...
Text Solution
|
- Is there any participation of the catalyst in the chemical process?
Text Solution
|
- How does a catalyst work?
Text Solution
|
- State the rate law of chemical reactions.
Text Solution
|