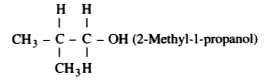
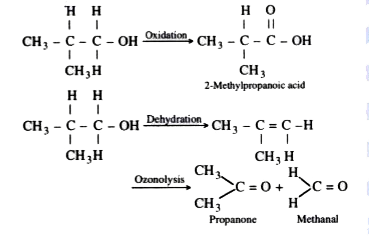
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-PROLEMS BASED ON CHEMICAL STRUCTURES AND REACTIONS -QUESTIONS
- An organic compound has the formula C4H100. It reacts with metallic so...
Text Solution
|
- 0.36 g of a bromoderivative of a hydrocarbon (A) when vaporized occupi...
Text Solution
|
- 0.037 g of an alcohol, ROH was added to CH3MgI and the gas evolved mea...
Text Solution
|
- Write the structures of all isomeric alcohols of molecular formula C4H...
Text Solution
|
- Anorganic compound A (molecular formula C4H10O) reacts vigorously with...
Text Solution
|
- Two isomers A and B having molecular formula C3H8O when passed separat...
Text Solution
|
- (i) An organic compound (P), C5H10O reacts with dil. H2SO4 to give (Q)...
Text Solution
|
- Anorganic compound with molecular formula C9H10O forms 2,4-DNP deriva...
Text Solution
|
- An organic compound (A) (molecular formula C(8)H(16)O(2)) was hydrolys...
Text Solution
|
- (a) Give chemical tests to distinguish between compounds in the follo...
Text Solution
|
- Compound 'A' was prepared by oxidation of oxidation of compound 'B' wi...
Text Solution
|
- An alkene 'A' (molecular formula C(5)H(10)) on ozonolysis gives a mixt...
Text Solution
|
- An aromatic compound 'A' (molecular formula C(8)H(8)O) gives positive ...
Text Solution
|
- When liquid 'A' is treated with a freshly prepared ammoniacal silver n...
Text Solution
|
- An organic compound 'A'on treatment with ammoniacal silver nitrate giv...
Text Solution
|
- Five isomeric p-subsituted aromatic compounds (A) to (E ) with molecul...
Text Solution
|
- An organic compound liberates CO2 on reaction with sodium bicarbonate....
Text Solution
|
- CH3 - overset(O)overset(||)C - OH overset(2RLi)toA , A overset(H3O^(+)...
Text Solution
|
- Anorganic compound with molecular formula C9H10O forms 2,4-DNP derivat...
Text Solution
|
- Compound A (C6H12O2) on reduction with LiAlH4, yielded two compounds B...
Text Solution
|