Text Solution
Verified by Experts
ICSE-SOME IMPORTANT ORGANIC NAME REACTIONS -Questions
- Acylation or Acetylation.
Text Solution
|
- Explain the following reactions : Aldol condensation
Text Solution
|
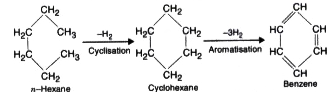
- Aromatisation
Text Solution
|
- Baeyer Villiger Oxidation of Ketones.
Text Solution
|
- Balz-Schiemann reaction.
Text Solution
|
- Benzoin Condensation.
Text Solution
|
- Bast's reaction
Text Solution
|
- Bergius process.
Text Solution
|
- Birch reduction.
Text Solution
|
- Birnbaum-Simonini reaction.
Text Solution
|
- Borodine - Hunsdiecker reaction is an example of:
Text Solution
|
- Beckmann rearrangement.
Text Solution
|
- Bouveault Blanc reduction.
Text Solution
|
- Cannizzaro's reaction
Text Solution
|
- Carbylamine reaction.
Text Solution
|
- Claisen Condensation.
Text Solution
|
- Claisen - Schmidt condensation
Text Solution
|
- Clemmensen reduction
Text Solution
|
- Coupling Reaction
Text Solution
|
- Corey-House reaction.
Text Solution
|