Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SOME IMPORTANT ORGANIC NAME REACTIONS -Questions
- Bouveault Blanc reduction.
Text Solution
|
- Cannizzaro's reaction
Text Solution
|
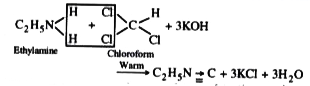
- Carbylamine reaction.
Text Solution
|
- Claisen Condensation.
Text Solution
|
- Claisen - Schmidt condensation
Text Solution
|
- Clemmensen reduction
Text Solution
|
- Coupling Reaction
Text Solution
|
- Corey-House reaction.
Text Solution
|
- Cristol reaction
Text Solution
|
- Cope rearrangement
Text Solution
|
- Darzen's process.
Text Solution
|
- Decarboxylation.
Text Solution
|
- Diazotisation
Text Solution
|
- Complete the following Deils - Alder reactions.
Text Solution
|
- Dow process.
Text Solution
|
- Elb's reaction
Text Solution
|
- Esterification Reaction or Fisher esterification.
Text Solution
|
- Etard's Reaction.
Text Solution
|
- E(2) reaction (Elimination bimolecular reaction)
Text Solution
|
- E(1) reaction (Elimination unimolecular reaction).
Text Solution
|