Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SOME IMPORTANT ORGANIC NAME REACTIONS -Questions
- Reimer - Tiemann reaction.
Text Solution
|
- Rosenmund's Reaction
Text Solution
|
- Define Raschig Process
Text Solution
|
- Sabatier-Senderen's reduction.
Text Solution
|
- Sandmeyer's reaction
Text Solution
|
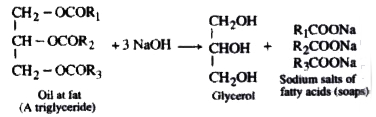
- Saponification
Text Solution
|
- Schmidt Reaction
Text Solution
|
- Schotten-Baumann Reaction.
Text Solution
|
- Stephen Reduction.
Text Solution
|
- Strecker's synthesis.
Text Solution
|
- Swarts reaction.
Text Solution
|
- Tischenko reaction.
Text Solution
|
- Trans - esterofication reaction
Text Solution
|
- Thermal Decomposition or Pyrolysis or Cracking.
Text Solution
|
- Ullmann Synthesis.
Text Solution
|
- Williamson's Synthesis
Text Solution
|
- Wurtz reaction.
Text Solution
|
- Wurtz - Fitting reaction
Text Solution
|
- Wolff rearrangement
Text Solution
|
- Wolff-Kishner reduction.
Text Solution
|