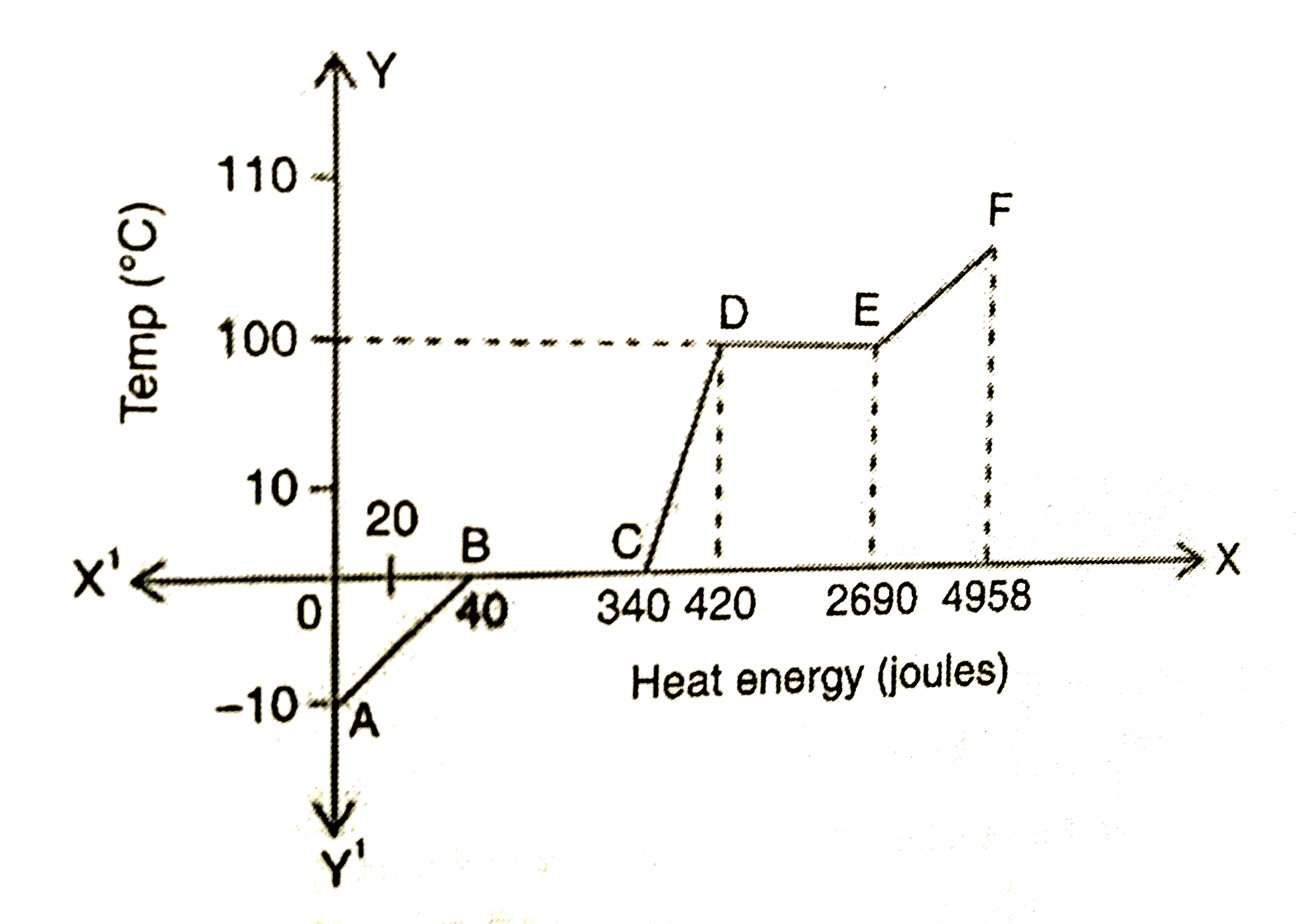A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- If mass of the substacne is 20 g then the heat energy required to melt...
Text Solution
|
- The nitrate which when heated gives off a gas (or) a mixture of gases ...
Text Solution
|
- The amount of heat energy required to increase the temperature of 20 g...
Text Solution
|
- The amount of heat energy required to increase the temperature of 20 g...
Text Solution
|
- The heating curve of a particular substance in solid state is as shows...
Text Solution
|
- For a substacne to undergo a change of state heat must be either give...
Text Solution
|
- The heating curve of a particular substance in solid state is a shown ...
Text Solution
|
- If mass of the substacne is 20 g then the heat energy required to melt...
Text Solution
|
- If Q cal of heat energy is supplied to 'm' kg of substacne its temepra...
Text Solution
|