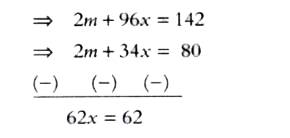Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PEARSON IIT JEE FOUNDATION-AIR AND OXYGEN-LEVEL-3
- The molecular masses of sulphate and hydroxide of a metal M are 142 an...
Text Solution
|
- 'Though oxygen is the supporter of life on earth, the presence of nitr...
Text Solution
|
- Why the position of freezer in the refrigerator is generally at the to...
Text Solution
|
- How does sky appear from the moon?
Text Solution
|
- Two liquids A and B were taken in two diffrernt barometer, the density...
Text Solution
|
- What would happen if people travel in a non-pressurized aeroplane?
Text Solution
|
- Electric bulbs are filled with argon gas but not air. Explain.
Text Solution
|
- The body temperature of a healthy person is around 98.4^(@)F. Justify.
Text Solution
|
- U.V rays find application in the purification of water, although it is...
Text Solution
|
- Deep sea divers carry oxygen diluted by helium in cylinders instead of...
Text Solution
|
- The temperature of troposphere decrease with increase in altitude. Is ...
Text Solution
|