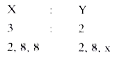A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The ratio of difference in the number of electrons between K and M she...
Text Solution
|
- The ratio of difference in the number of electrons between K and M sh...
Text Solution
|
- The difference in the number of elelctrons between K, L and L, M shell...
Text Solution
|
- The ratio of difference in the number of electrons between K and M she...
Text Solution
|
- The difference in the number of elelctrons between K, L and L, M shell...
Text Solution
|
- Two elements X and Y have 6 and 7 electrons in their N-shell and M-she...
Text Solution
|
- (i) Element X has two valence electrons in M-shell . (ii) In elemen...
Text Solution
|
- तत्व X का इलेक्ट्रॉनिक विन्यास है - {:(K,L,M),(2,8,6):} X के परमाण...
Text Solution
|
- Elements with stable electronic configurations have eight electrons in...
Text Solution
|