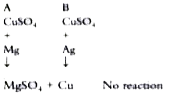Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- In a science fair , a student took two test tubes labelled A and B fi...
Text Solution
|
- A student took four test tubes containing solutions of different colou...
Text Solution
|
- A student took four test tubes I,II,III and IV containing aluminium su...
Text Solution
|
- A student addd few pieces of aluminium metal to two test tubes A and B...
Text Solution
|
- Four test tubes were taken and marked A, B, C and D respectively. 2 mL...
Text Solution
|
- In a science fair , a student took two test tubes labelled A and B fi...
Text Solution
|
- A student addd few pieces of aluminium metal to two test tubes A and B...
Text Solution
|
- A student was asked to take 10 ml of rainwater in test tube A and l g ...
Text Solution
|
- Mohan takes 5 mL of distilled water in four test tubes A, B, C and D. ...
Text Solution
|