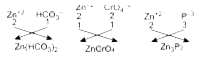Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- An atom of an element 'X' has three completely filled shell . The ra...
Text Solution
|
- An atom of an element has the electrons distributed in four shells . I...
Text Solution
|
- An atom of an element 'X' has three completely filled shell . The rati...
Text Solution
|
- An atom of an element has its K and L shells completely filled with el...
Text Solution
|
- An atom has its K and L shells completely filled and seven electrons i...
Text Solution
|
- An atom has its K and L shells completely filled and five electrons in...
Text Solution
|
- There are three shells in the atom of element 'X', 6 electrons are pre...
Text Solution
|
- There are three shells in the atom of element 'X', 6 electrons are pre...
Text Solution
|
- There are three shells in the atom of element 'X', 6 electrons are pre...
Text Solution
|