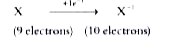A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- If overline(X) ion has 10 neutrons and 9 protons , then the electronic...
Text Solution
|
- If a uninegative ion has 10 neutrons and 9 protons , electronic con...
Text Solution
|
- If overline(X) ion has 10 neutrons and 9 protons , then the electronic...
Text Solution
|
- A metallic element forms an ion with unit charge. The ion has 10 elect...
Text Solution
|
- किसी तत्व के परमाणु में 29 इलेक्ट्रॉन और 25 न्यूट्रॉन हैं इसमें प्र...
Text Solution
|
- किसी तत्व को .(19)^(40)X के रूप में प्रदर्शित किया जाता है। इस परमाणु...
Text Solution
|
- x^(2-) আয়নে 10 টী ইলেকট্রন ও 8 টি নিউট্রন আছে । মৌলটির পরমানু- ক্রমাঙ্...
Text Solution
|
- X^2- আয়নে 10 টি ইলেকট্রন ও 8 টি নিউট্রন আছে । মৌলটির পরমাণু-ক্রমাঙ্ক ...
Text Solution
|
- A metallic element forms an ion with unit charge. The ion has 10 elect...
Text Solution
|