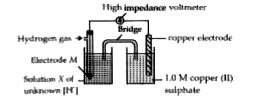A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
MTG GUIDE|Exercise NEET Café (Topicwise Practice Questions) (NERNST EQUATION) |8 VideosELECTROCHEMISTRY
MTG GUIDE|Exercise NEET Café (Topicwise Practice Questions) (CONDUCTANCE OF ELECTROLYTIC SOLUTIONS) |42 VideosELECTROCHEMISTRY
MTG GUIDE|Exercise AIPMT/NEET(MCQs) |5 VideosCOORDINATION COMPOUNDS
MTG GUIDE|Exercise CHECK YOUR NEET VITALS|30 VideosGENERAL PRINCIPALS AND PROCESSES OF ISOLATION OF ELEMENTS
MTG GUIDE|Exercise AIPMT/NEET (MCQs)|10 Videos
Similar Questions
Explore conceptually related problems
MTG GUIDE-ELECTROCHEMISTRY-NEET Café (Topicwise Practice Questions) (CELLS AND ELECTRODE POTENTIAL)
- Two weak acid solutions HA1 and HA2 each with the same concentration a...
Text Solution
|
- The following facts are available: 2A^+B2to2B^-+A2 2C^(-)+B2to No ...
Text Solution
|
- A student set up the following apparatus to determine the hydrogen ion...
Text Solution
|
- Concentration polarization arises because of the
Text Solution
|
- Which of the following statement is incorrect?
Text Solution
|
- An aqueous solution containing one mole per litre each Cu(NO3)2, AgNO3...
Text Solution
|
- A gas z is bubbled through a solution containing x^- and y^- . If the ...
Text Solution
|
- A student made the following observations in the laboratory (i) Clea...
Text Solution
|
- Indicator electrode is
Text Solution
|
- In an electrochemical cell, the electrons flow from
Text Solution
|
- Saturated solution of KNO3 , is used to make 'salt bridge because
Text Solution
|
- Electrode potentials (E(red)^@) of 4 elements A, B, C and D are -1.36,...
Text Solution
|
- Which one of the following does not hold good for S.H.E?
Text Solution
|
- For the electrochemical cell having E^@(M^+//M) =0.44 V and E^@(X//X...
Text Solution
|
- The reference calomel electrode is made from which of the following?
Text Solution
|
- Out of Cu, Ag, Fe and Zn, the metal which can displace all others from...
Text Solution
|
- If half-cell reaction A+e^(-)toA^- has a large negative reduction pote...
Text Solution
|
- Which of the substances Na, Hg, S, Pt and graphite can be used as elec...
Text Solution
|
- In a Galvanic cell
Text Solution
|
- Electrode potential data for a few elements is given Fe((aq))^(3+)+e...
Text Solution
|