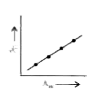
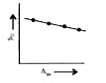
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
MTG GUIDE|Exercise NEET Café (Topicwise Practice Questions) (ELECTROLYTIC CELLS AND ELECTROLYSIS) |94 VideosELECTROCHEMISTRY
MTG GUIDE|Exercise NEET Café (Topicwise Practice Questions) (BATTERIES, FUEL CELLS AND CORROSION) |6 VideosELECTROCHEMISTRY
MTG GUIDE|Exercise NEET Café (Topicwise Practice Questions) (NERNST EQUATION) |8 VideosCOORDINATION COMPOUNDS
MTG GUIDE|Exercise CHECK YOUR NEET VITALS|30 VideosGENERAL PRINCIPALS AND PROCESSES OF ISOLATION OF ELEMENTS
MTG GUIDE|Exercise AIPMT/NEET (MCQs)|10 Videos
Similar Questions
Explore conceptually related problems
MTG GUIDE-ELECTROCHEMISTRY-NEET Café (Topicwise Practice Questions) (CONDUCTANCE OF ELECTROLYTIC SOLUTIONS)
- Molar conductances of BaCl2 , H2SO4 and HCl at infinite dilutions are ...
Text Solution
|
- An increase in equivalent conductance of a strong electrolyte with dil...
Text Solution
|
- Which of the following curve gives the variation of Lambdam with sqrt...
Text Solution
|
- The conductivity of 0.001028 mol L^(-1) acetic acid is 4.95xx10^(-5) ...
Text Solution
|
- The molar ionic conductivities of NH4^+ and OH^(-) at infinite dilutio...
Text Solution
|
- An electrolyte
Text Solution
|
- Pure water does not conduct electricity because it
Text Solution
|
- A cell constant is generally found by measuring the conductivity of aq...
Text Solution
|
- Which of the following is a strong electrolyte?
Text Solution
|
- The unit of equivalent conductivity is
Text Solution
|
- The resistance of 1 N solution of acetic acid is 250 ohm, when measure...
Text Solution
|
- The specific conductance of 0.1 M NaCl solution is 1.06xx10^(-2) ohm^(...
Text Solution
|
- Molar ionic conductivities of a bivalent electrolyte are 57 and 73. Th...
Text Solution
|
- The unit of specific conductivity is
Text Solution
|
- The specific conductance of a 0.1 N KCl solution at 23 "^@C is 0.012 o...
Text Solution
|
- At infinite dilution in the aqueous solution of BacI2, molar conductiv...
Text Solution
|
- Specific conductivity of a solution
Text Solution
|
- The substance having highest conductivity at room temperature among th...
Text Solution
|
- Molar conductivity of a solution is 1.26xx10^(2) ohm^(-1) cm^(2) mol^(...
Text Solution
|
- What is the cell constant of a cell of KCl containing N/50 solution if...
Text Solution
|