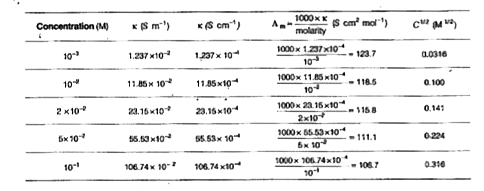
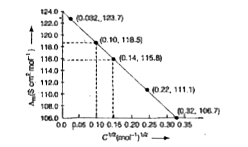
Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
ARIHANT PUBLICATION|Exercise QUESTIONS FOR ASSESSMENT ( PART II CONDCUTIVITY OF SOLUTIONS AND KOHLROUSCH.S LAW) ( MULTIPLE CHOICE TYPE QUESTIONS)|2 VideosELECTROCHEMISTRY
ARIHANT PUBLICATION|Exercise QUESTIONS FOR ASSESSMENT ( PART II CONDCUTIVITY OF SOLUTIONS AND KOHLROUSCH.S LAW) ( VERY SHORT ANSWER TYPE QUESTIONS)|3 VideosELECTROCHEMISTRY
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (PART II) ( CONDUCTIVITY OF SOLUTION AND KOHLRAUSCH.S LAW) ( SHORT ANSWER TYPE II QUESTIONS )|5 VideosD-BLOCK ELEMENTS
ARIHANT PUBLICATION|Exercise Chapter practice (Long answer type questions)|2 VideosELEMENTS : NITROGEN FAMILY
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE ( Long Answer Type Questions ) |11 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-ELECTROCHEMISTRY-QUESTIONS FOR PRACTICE (PART II) ( CONDUCTIVITY OF SOLUTION AND KOHLRAUSCH.S LAW) ( LONG ANSWER TYPE QUESTIONS )
- Define the equivalent conductance and specific conductance.
Text Solution
|
- Discuss the effect of dilution on these conductance of an electrolyte ...
Text Solution
|
- The specific conductance of a decinormal solution of NaCl equals to 0....
Text Solution
|
- Define conductivity and molar conductivity for the solution of an elec...
Text Solution
|
- Conductivity of 0.00241M acetic acid is 7.896 xx 10^(-5) S cm^(-1). Ca...
Text Solution
|