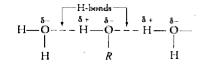Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (Short Answer Type I Questions)|8 VideosALCOHOLS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (Short Answer Type II Questions)|15 VideosALCOHOLS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (Long Answer Type Questions)|4 VideosAMINES
ARIHANT PUBLICATION|Exercise Chapter Practice (Long Answer Type Questions)|2 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-ALCOHOLS -QUESTIONS FOR PRACTICE (Multiple Choice Type Questions)
- Hydroboration-oxidation of propene yield.
Text Solution
|
- Boiling point of alcohol is comparatively higher than that of correspo...
Text Solution
|
- Convert of ethyl alcohol into acetaldehyde is an example of
Text Solution
|
- Catalytic dehydrogenation of a primary alcohol gives a/an
Text Solution
|
- Identify the allylic alcohols in the following examples.
Text Solution
|
- Write the IUPAC name of the compound given below CH(3)-CH(2)- under...
Text Solution
|
- Of the two hydroxy organic compounds, ROH and R'OH. The first one is b...
Text Solution
|
- Lower alcohols are water soluble whereas higher alcohols are water ins...
Text Solution
|
- Alcohols are comparatively more soluble in water than hydrocarbons of ...
Text Solution
|
- Esterification does not take place between ethyl alcohol and excess H(...
Text Solution
|
- Name the alcohol that is used to make the following ester: CH(3)- ov...
Text Solution
|
- Give the order of dehydration for primary, secondary and tertiary alco...
Text Solution
|
- The most suitable reagent for the conversion of RCH(2) OH to RCHO is
Text Solution
|
- Suggest a reagent for the following conversion:
Text Solution
|
- The alcohols propan-1-ol and propan-2-ol are distinguished by............
Text Solution
|