Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS
ARIHANT PUBLICATION|Exercise ODISHA BUREAU.S TEXTBOOK SOLUTIONS (Long Answer Type Questions )|14 VideosALCOHOLS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE ( MULTIPLE CHOICE TYPE QUESTIONS )|3 VideosALCOHOLS
ARIHANT PUBLICATION|Exercise ODISHA BUREAU.S TEXTBOOK SOLUTIONS ( Short Answer Type I Questions )|29 VideosAMINES
ARIHANT PUBLICATION|Exercise Chapter Practice (Long Answer Type Questions)|2 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-ALCOHOLS -ODISHA BUREAU.S TEXTBOOK SOLUTIONS (Short Answer Type II Questions )
- How are the alkanols classified? Give an example of each class with th...
Text Solution
|
- There are three unlabelled bottles containing methyl alcohol, ethyl al...
Text Solution
|
- How will you convert methanol to ethanol and vice-versa?
Text Solution
|
- How many different isomeric alcohols having molecular formula, C(4)H(1...
Text Solution
|
- Ethyl alcohol (A) reacts with conc. H(2)SO(4) at different temperatur...
Text Solution
|
- An organic compound gives hydrogen on reacting with sodium metal. It a...
Text Solution
|
- How will you convert ethanol to iodoform and chloroform?
Text Solution
|
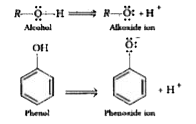
- Explain why Ethanol is less acidic than phenol.
Text Solution
|
- Explain why Though dimethyl ether and ethanol are isomers, still the...
Text Solution
|