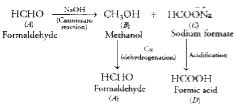
Text Solution
Verified by Experts
Topper's Solved these Questions
CARBOXYLIC ACIDS
ARIHANT PUBLICATION|Exercise PART I QUESTIONS FOR PRACTICE (LONG ANSWER TYPE QUESTIONS) |4 VideosCARBOXYLIC ACIDS
ARIHANT PUBLICATION|Exercise PART I QUESTIONS FOR ASSESSMENT (MCQ)|2 VideosCARBOXYLIC ACIDS
ARIHANT PUBLICATION|Exercise PART I QUESTIONS FOR PRACTICE (SHORT ANSWER TYPE I QUESTIONS) |2 VideosARYL DIAZONIUM SALTS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS) 7 MARKS |2 VideosCHEMICAL KINETICS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (Long Answer Type Questions)|8 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-CARBOXYLIC ACIDS -PART I QUESTIONS FOR PRACTICE (SHORT ANSWER TYPE II QUESTIONS)
- An organic compound A (molecular formula (C(8)H(16)O(2)) was hydrolyse...
Text Solution
|
- Two moles of an organic compound A on treatment with a strong base giv...
Text Solution
|
- Show the preparation of benzoic acid from toluene
Text Solution
|
- Show the preparation of benzoic acid from carboxylation of Grignard'...
Text Solution
|
- How do you prepare benzoic acid from n-propyl benzene
Text Solution
|
- How do you prepare benzoic acid from phenylcyanide
Text Solution
|
- How can you get benzoic acid from benzene?
Text Solution
|